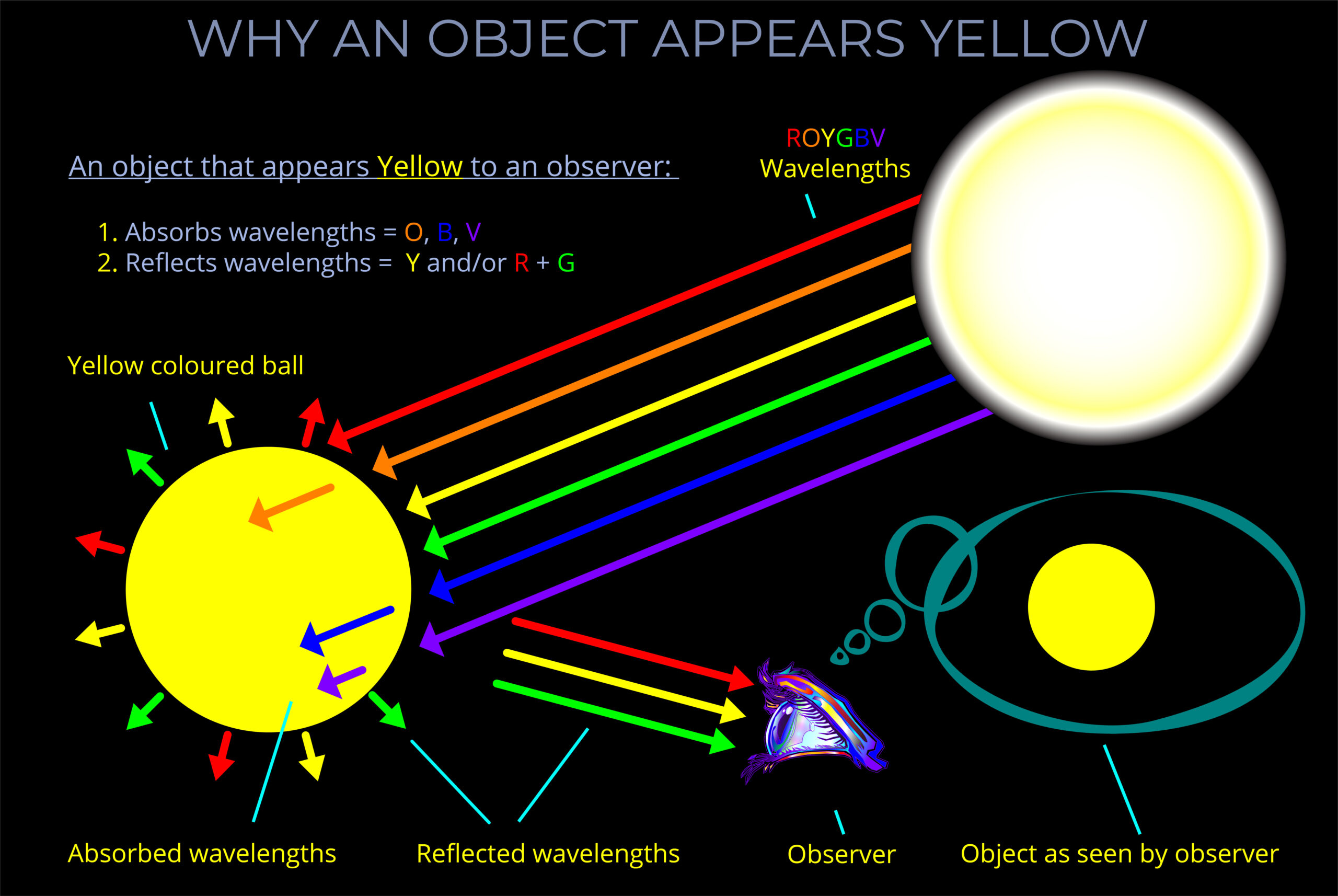
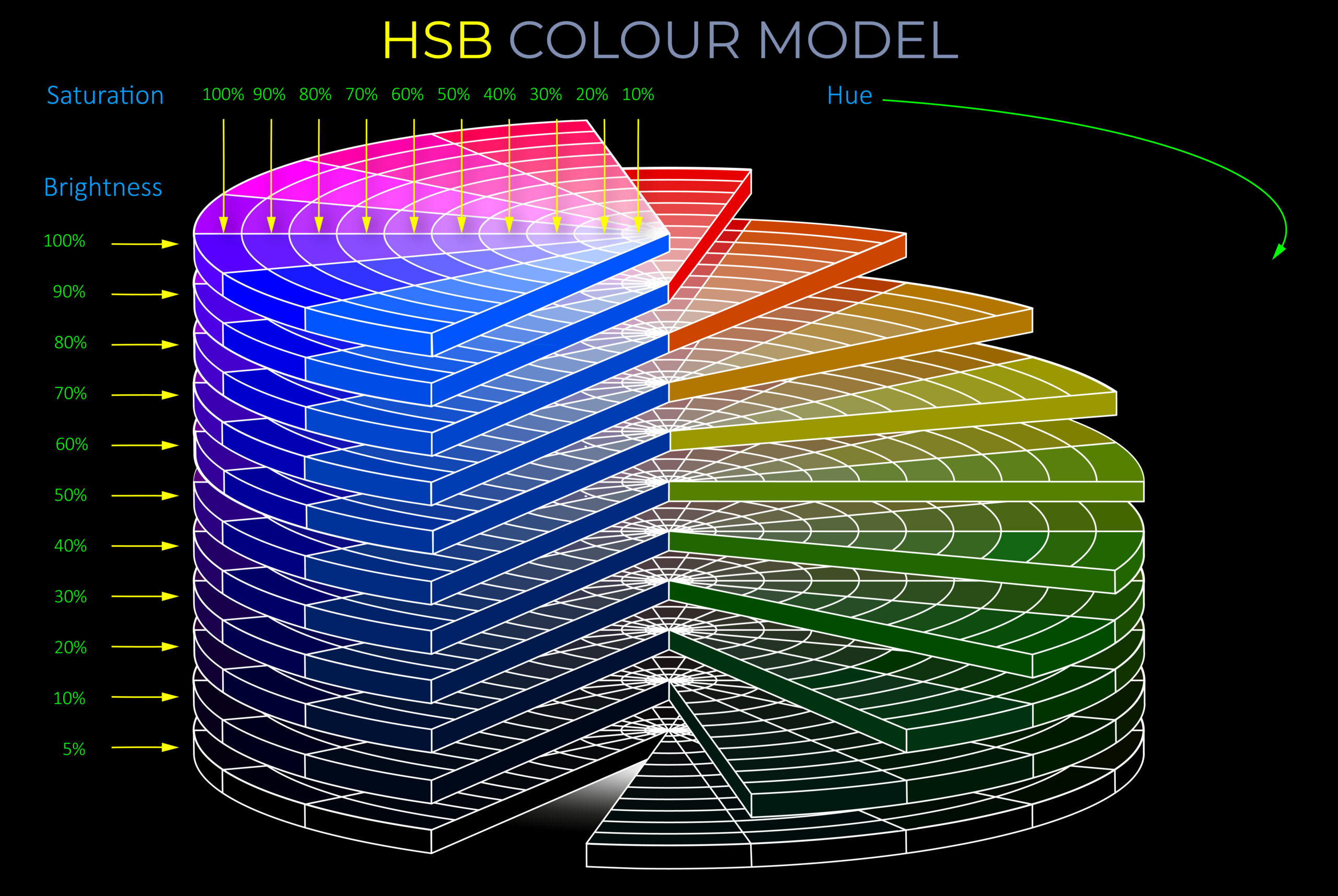
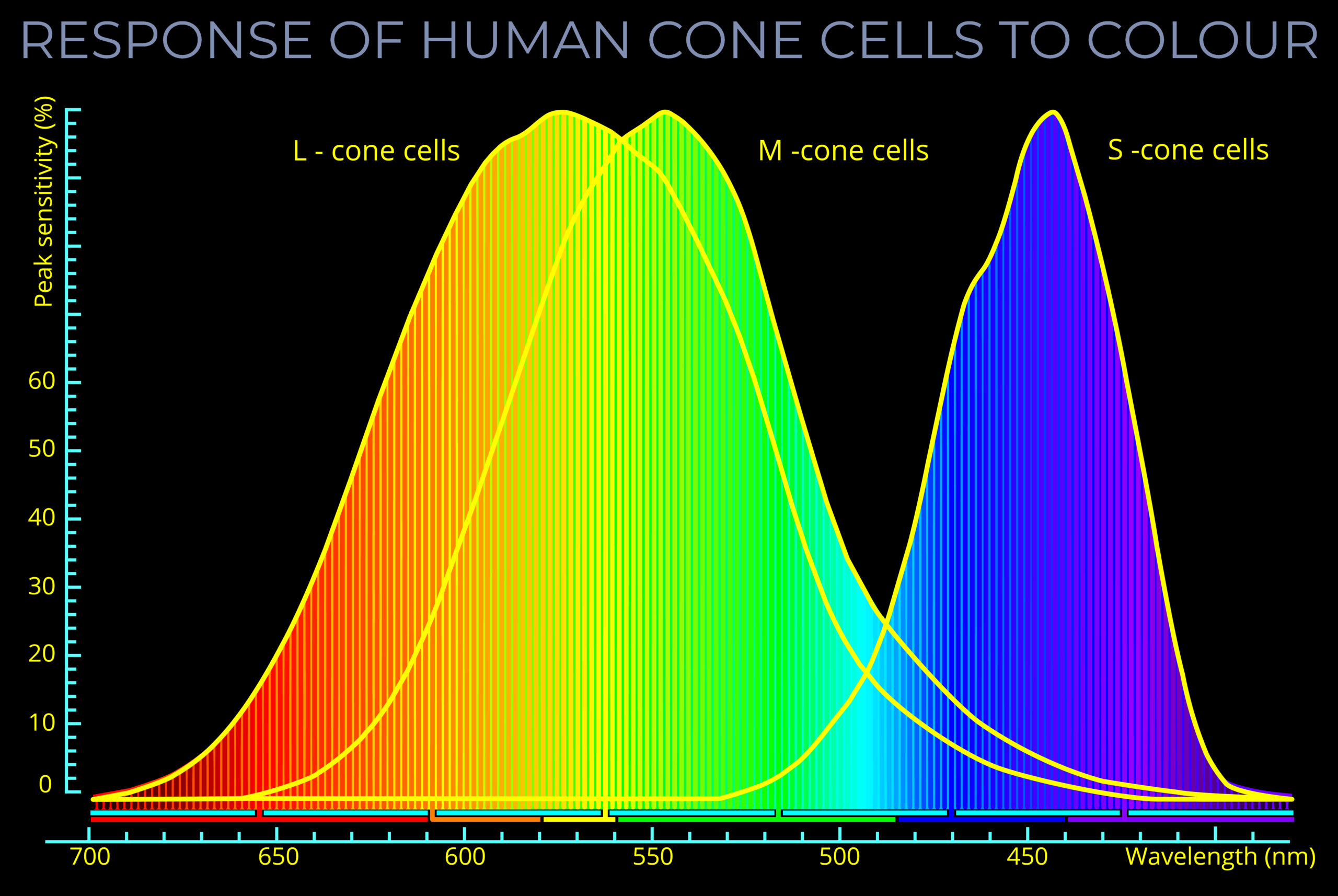
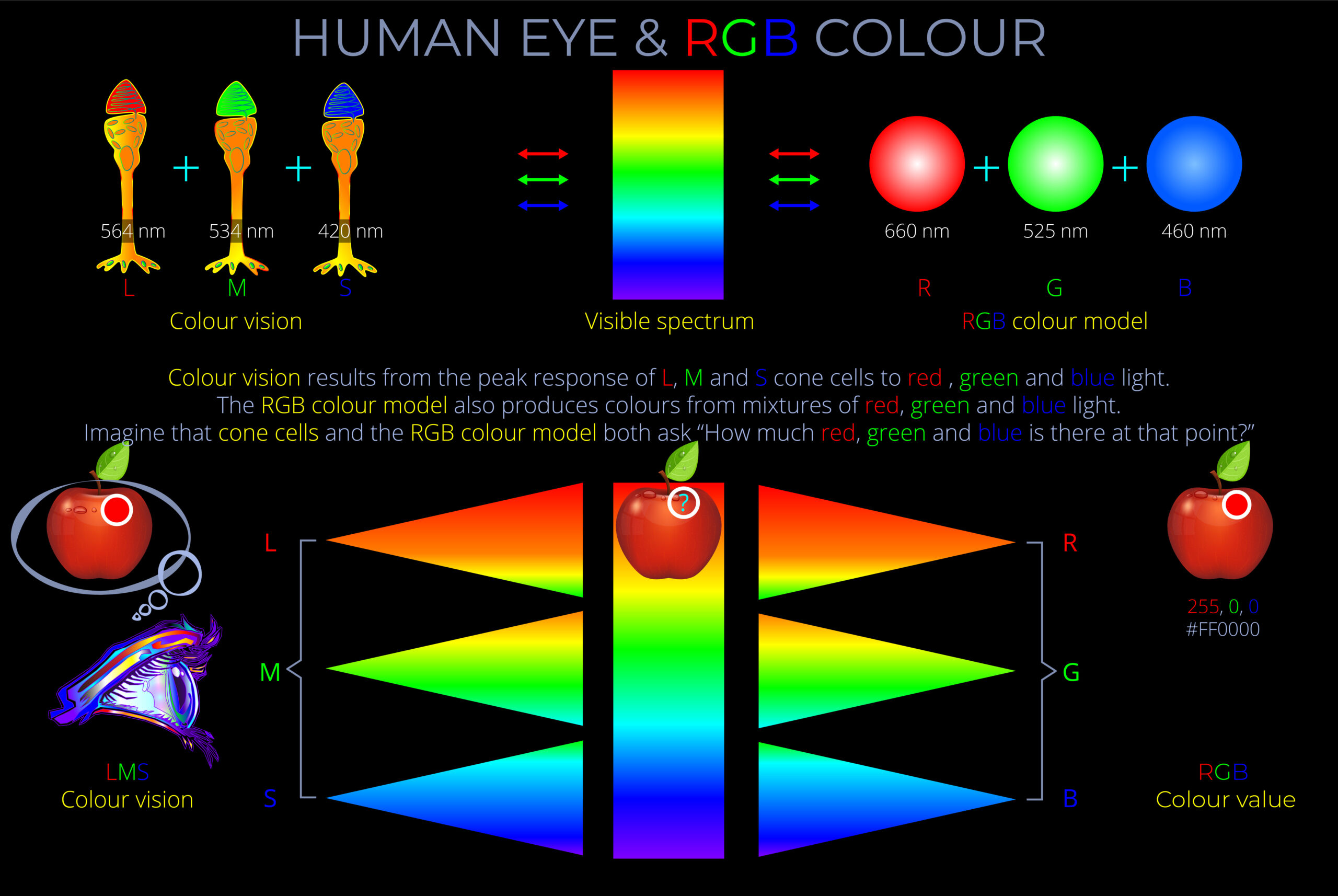
| | | |
| LIGHT-EMITTING PROCESS | | | |
| Luminescence | Light emission due to the excitation of electrons in a material. | Electrons within a material gain energy and then release light as they return to a lower energy state. | Bioelectroluminescence
Electroluminescence
Photoluminescence
- Fluorescence
- Phosphorescence
Sonoluminescence
Thermoluminescence
|
| Blackbody radiation (Type of thermal radiation) | Electromagnetic radiation (including visible light) emitted by any object with a temperature above absolute zero. | Electromagnetic radiation (including visible light) emitted by any object with a temperature above absolute zero. | All objects above temperature of absolute zero. |
| Chemiluminescence | Light from natural and artificial chemical reactions. | Light from natural and artificial chemical reactions. | Bioluminescence
Chemiluminescent reactions:
- Luminol reactions
- Ruthenium chemiluminescence |
| Nuclear reaction | Light emission as a byproduct of nuclear reactions (fusion or fission). | Light emitted as a byproduct of nuclear reactions. | Nuclear reactors
Stars undergoing fusion |
| Thermal radiation | Light emission due to the thermal excitation of atoms and molecules at high temperatures. | Light emission due to the thermal excitation of atoms and molecules. | Sun
Stars
Incandescent light bulbs |
| Triboluminescence | Light emission due to mechanical stress applied to a material. | Light emission due to the mechanical stress applied to a material, causing the movement of electric charges and subsequent light emission. | Sugar crystals cracking
Adhesive tape peeling
Quartz crystals fracturing. |
| | | |
| Natural light source | | | |
Fireflies
Deep-sea creatures
Glowing mushrooms | Bioluminescence | Light emission from biological organisms. | Involves the luciferase enzyme. |
Sun
Stars | Nuclear Fusion | Light emission as a byproduct of nuclear fusion reactions in stars. | Electromagnetic spectrum (visible light, infrared, ultraviolet). |
Fire
Candles | Thermal radiation | Light emission due to the thermal excitation of atoms and molecules during the combustion of a fuel source. | Burning of a fuel source, releasing heat and light. |
| Artificial light source | | | |
Fluorescent lights Highlighters
Safety vests | Chemiluminescence | Light emission from chemical reactions. | Fluorescence (absorption and re-emission of light). |
Glow sticks
Emergency signs | Chemiluminescence | Light emission due to phosphorescence - a type of chemiluminescence. | A type of chemiluminescence where light emission is delayed after the initial excitation. |
Glow sticks
Light sticks | Chemiluminescence | Chemiluminescence | Light emission from a chemical reaction that does not involve combustion. |
Tungsten light bulbs
Toasters | Thermal radiation | Heated filament radiates light and heat. | Light emission from a hot filament. |
Fluorescent lamps
LED lights | Electroluminescence | Excitation of atoms by electric current. | Light emission when electric current excites atoms in a material. |
| Neon signs | Electrical Discharge | Discharge of electricity through gas. | Light emission when electricity flows through a gas. |
Sugar crystals cracking
Pressure-sensitive adhesives | Triboluminescence | Light emission from friction or pressure. | Light emission due to mechanical forces. |
Fluorescent paint Highlighters
Safety vests | Photoluminescence | Absorption and subsequent re-emission of light at a lower energy. | Absorption and re-emission of light. |