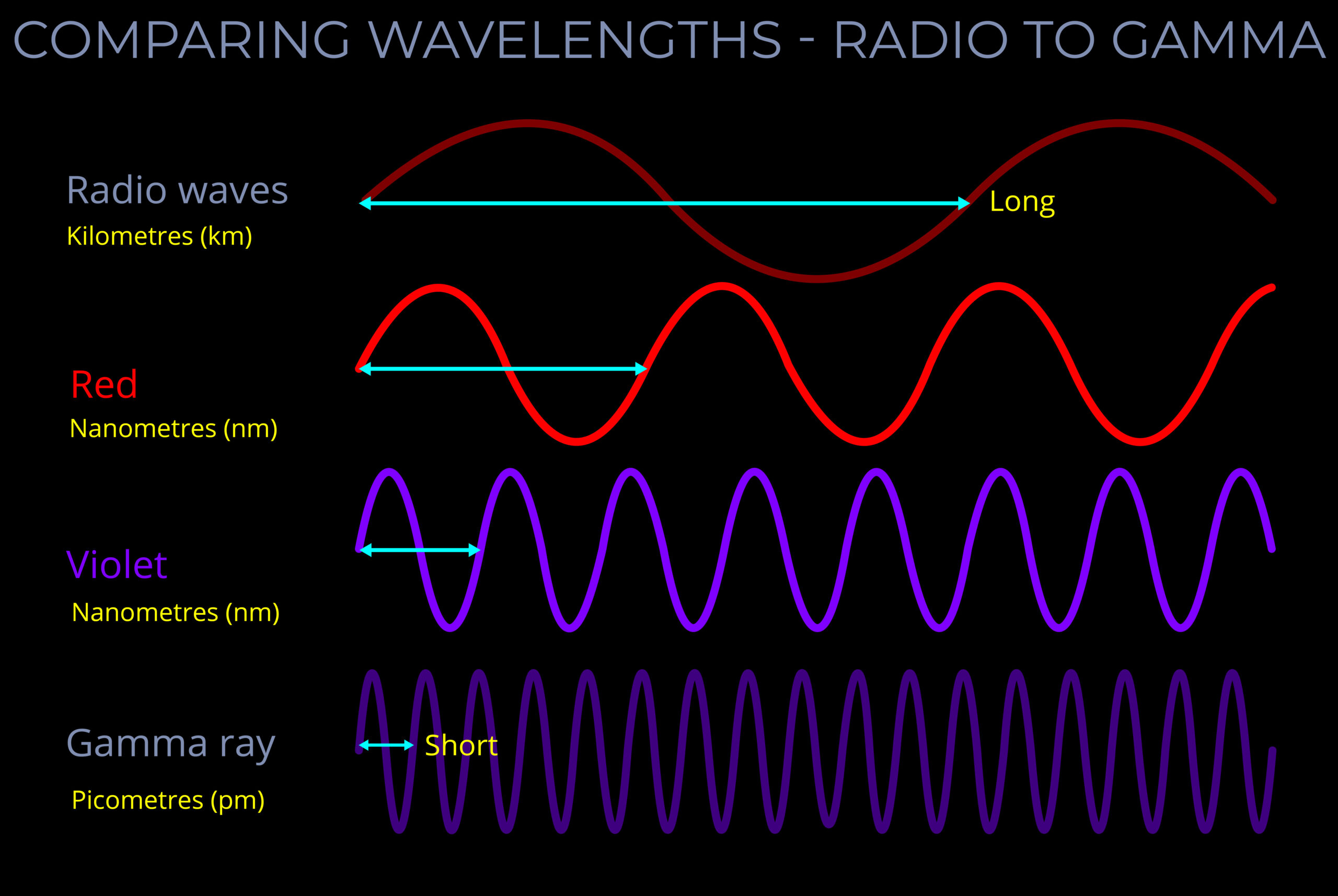
Wavelength is the distance from any point on a wave to the corresponding point on the next wave. This measurement is taken along the middle line of the wave.
- While wavelength can be measured from any point on a wave, it is often simplest to measure from the peak of one wave to the peak of the next, or from the bottom of one trough to the bottom of the next, ensuring the measurement covers a whole wave cycle.
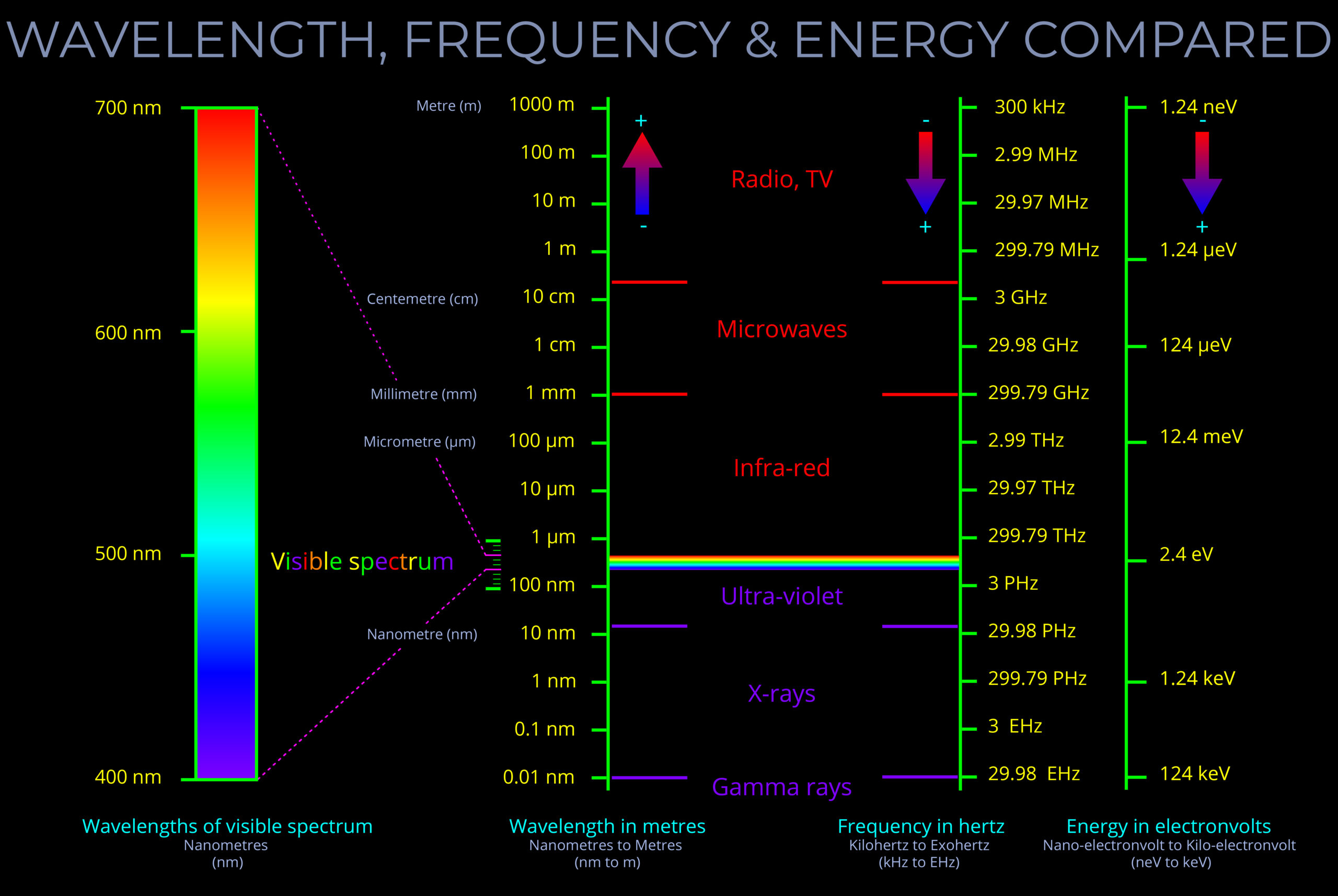
- The wavelength of an electromagnetic wave is usually given in metres.
- The wavelength of visible light is typically measured in nanometres, with 1,000,000,000 nanometres making up a metre.
- Each type of electromagnetic radiation – such as radio waves, visible light, and gamma waves – corresponds to a specific range of wavelengths on the electromagnetic spectrum.
- As energy increases, frequency also increases and the wavelength decreases. Therefore, shorter wavelengths correspond to higher energy levels, and longer wavelengths correspond to lower energy levels.
Wavelength & the Visible Spectrum
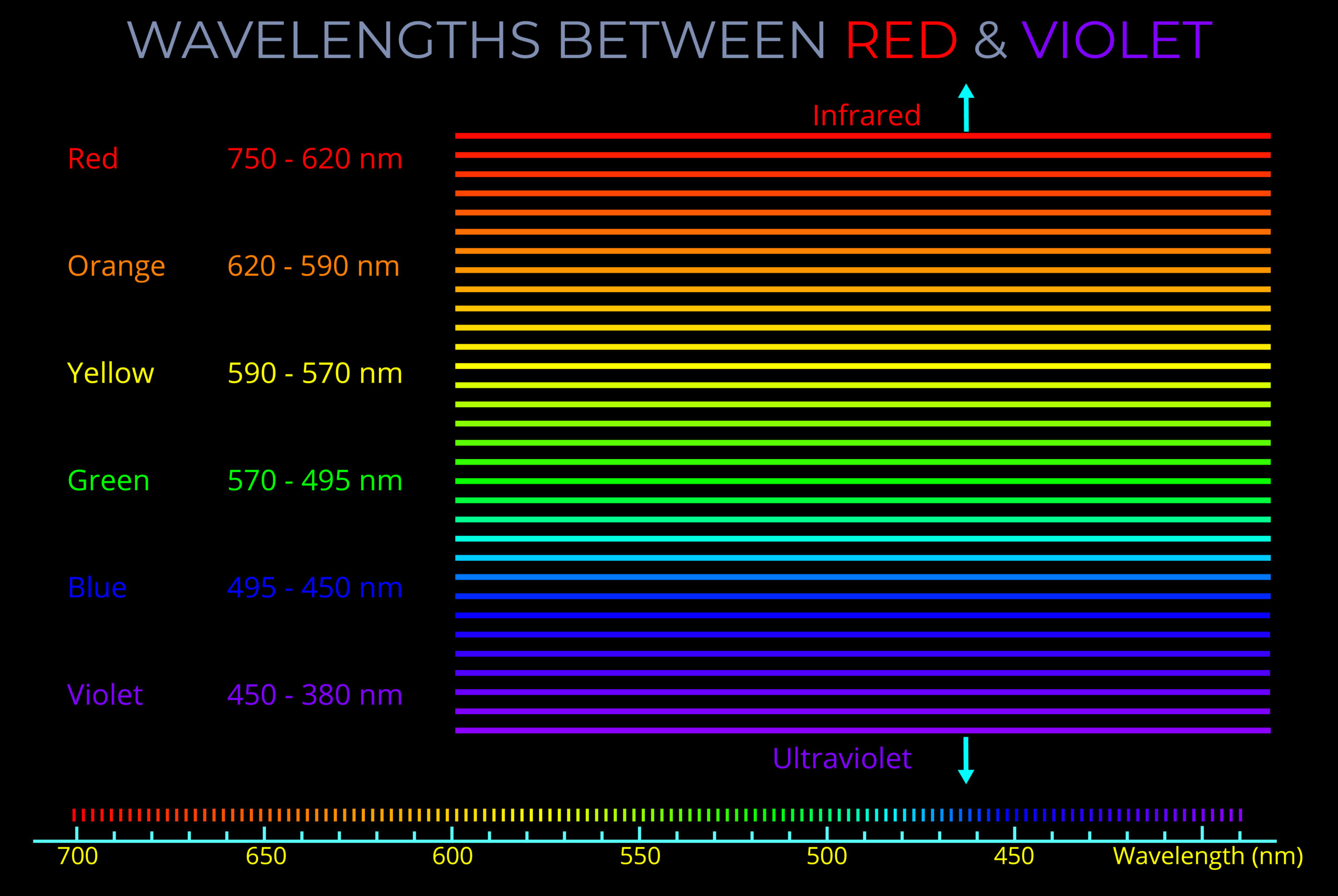
- The visible part of the electromagnetic spectrum consists of a range of wavelengths that correspond with all the different colours we see in the world.
- The visible spectrum, which spans wavelengths from approximately 400 to 700 nanometres, is commonly described in terms of bands of colour—red, orange, yellow, green, blue, indigo, and violet.
- However, these divisions are somewhat arbitrary since the spectrum is continuous, and the exact wavelength boundaries between colours is subjective.
- Humans don’t see the wavelengths of visible light directly but do see the specific colour associated with each wavelength and the colour produced when different wavelengths mix.
- Within this visible spectrum, each colour corresponds to a single wavelength of light.
- The concept of colour is a perceptual property, not a property of light itself.
- It’s how our brain interprets the wavelengths of light that reach our eyes. The perceived colour (hue) of a light stimulus is dependent on its wavelength.
- A colour produced by a single wavelength is known as a pure spectral colour.
Wavelength & Colour
- A light that we perceive as white is a combination of many different wavelengths of light across the visible spectrum. When these wavelengths containing red, green, and blue mix and reflect off a neutral-coloured surface, they appear white to our eyes.
- Light sources in nature rarely emit a single wavelength. They typically emit a mixture of many wavelengths that combine to create the colour we perceive upon reflection. The wider the range of wavelengths present in the light source, the less saturated (more diluted) the reflected colour will appear. In other words, light with a mix of many wavelengths tends to appear lighter or closer to white.
- Wavelengths of visible light are typically measured in nanometers (nm). While the human eye cannot distinguish every single possible wavelength within the visible spectrum (between 400 nm and 700 nm), it can differentiate a vast range of colours within that spectrum.
References
- Wavelength is the distance from any point on a wave to the corresponding point on the next wave. This measurement is taken along the middle line of the wave.
- While wavelength can be measured from any point on a wave, it is often simplest to measure from the peak of one wave to the peak of the next, or from the bottom of one trough to the bottom of the next, ensuring the measurement covers a whole wave cycle.
- The wavelength of an electromagnetic wave is usually given in metres.
- The wavelength of visible light is typically measured in nanometres, with 1,000,000,000 nanometres making up a metre.
- Each type of electromagnetic radiation – such as radio waves, visible light, and gamma waves – corresponds to a specific range of wavelengths on the electromagnetic spectrum.