All forms of light within the electromagnetic spectrum can be thought of as electromagnetic waves produced by the interplay of their electric and magnetic fields.
- Electromagnetic waves transport electromagnetic energy.
- The energy carried by electromagnetic waves is often simply called radiant energy or light.
- Electromagnetic radiation can also be described in terms of elementary particles called photons. Photons are energy packets and the quantum of the electromagnetic field.
- Electromagnetic waves represent a form of energy, which is emitted and absorbed by charged particles.
- We can sense electromagnetic waves transferring their energy when sunlight heats our skin.
- The energy that electromagnetic waves transport is linked to their frequency and wavelength. Greater energy corresponds to a higher frequency and shorter wavelength.
- Electromagnetic waves do not require a medium to propagate, hence they can travel through a vacuum.
- Electromagnetic waves are transverse waves, meaning their oscillations are perpendicular to the direction of propagation.
Illustrations of electromagnetic waves
- Electromagnetic waves can be visualized as synchronized oscillations of electric and magnetic fields that propagate at the speed of light in a vacuum.
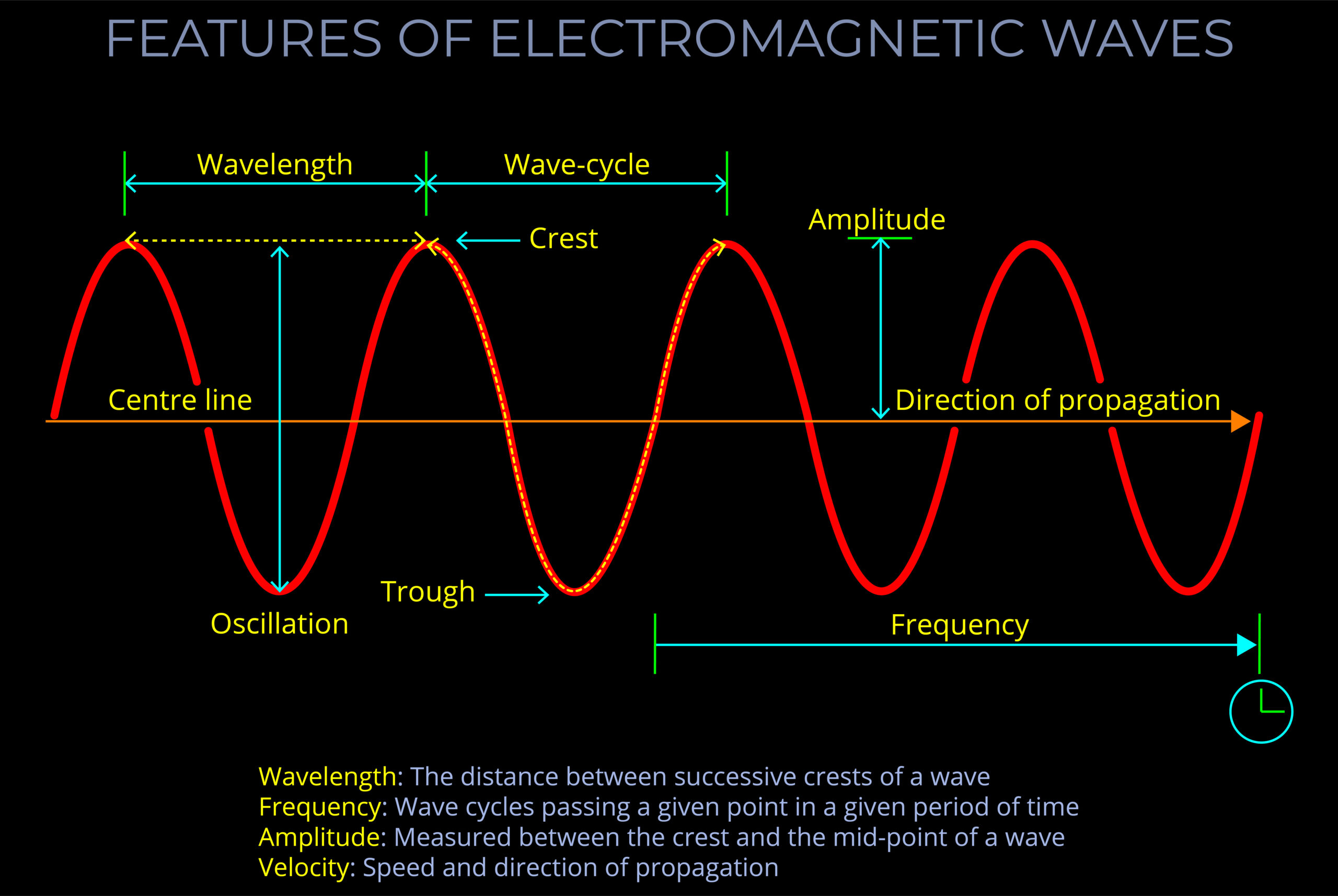
- Illustrations of electromagnetic waves may highlight:
- Velocity (v): Measures the rate and direction a wave moves in a specific medium.
- Velocity represents the speed and direction at which an electromagnetic wave propagates through a medium. It is typically constant for a given medium and is unrelated to wavelength, frequency, or amplitude.
- Wavelength (λ): The distance over which the shape of a wave repeats. Wavelength is measured in meters or sub-units of metres.
- wavelength is inversely related to frequency (), according to the equation . This means that as the wavelength increases, the frequency decreases, and vice versa.
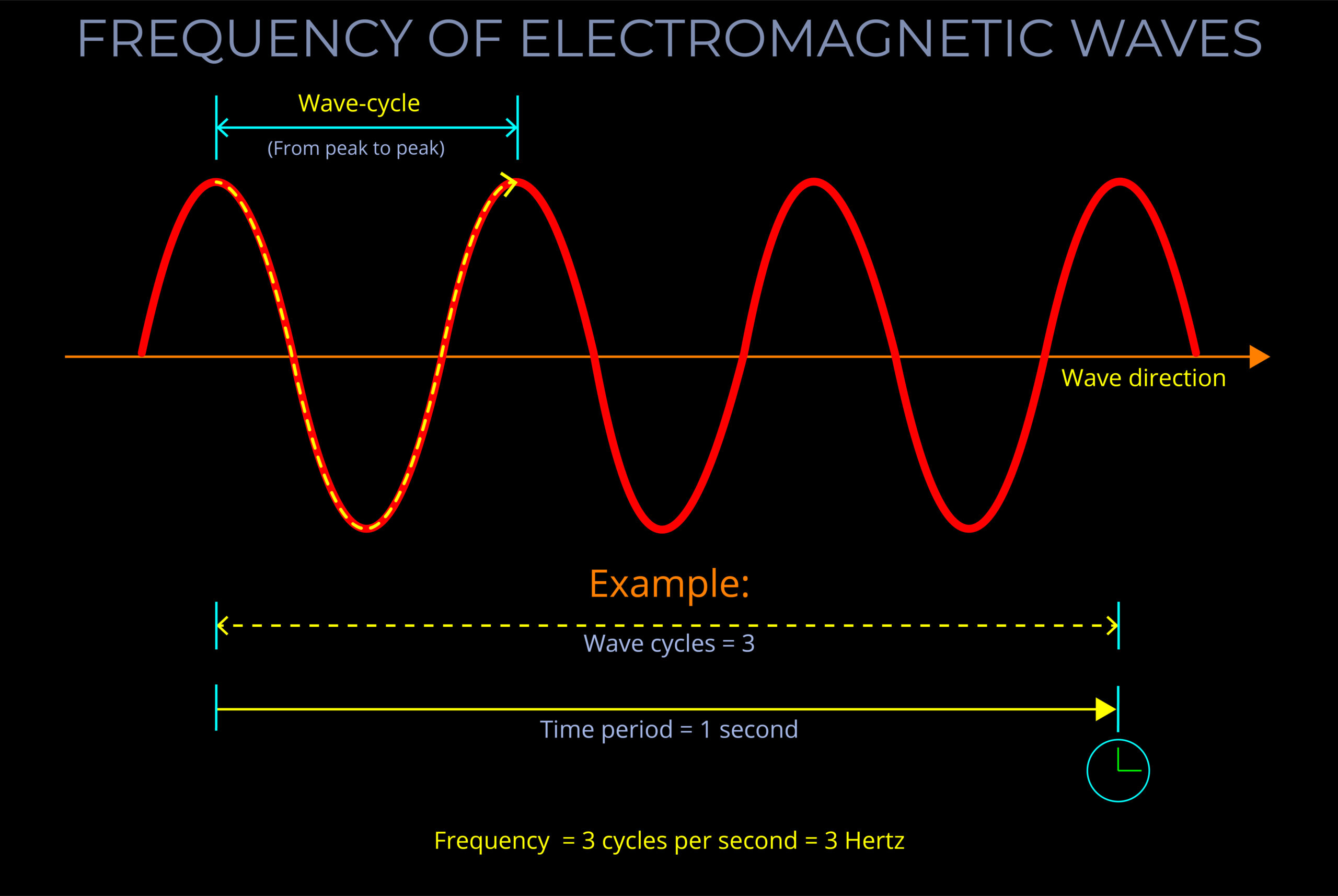
- Frequency (f): Frequency is measured in cycles per second. The unit of frequency is Hertz.
- Amplitude (A): Amplitude is the maximum magnitude of the wave’s oscillations. It represents the peak value of the wave’s electric and magnetic fields as they oscillate in space.
- Velocity (v): Measures the rate and direction a wave moves in a specific medium.
Electromagnetic waves consist of coupled oscillating electric and magnetic fields orientated at 900 to one another. (Credit: https://creativecommons.org/licenses/by-sa/4.0)
About the properties of photons
- A photon is a type of elementary particle that is a quantum (plural = quanta) of the electromagnetic field. This means that it is the smallest quantity into which light can be divided.
- A photon carries energy and can be described both in terms of a particle and a wave.
- While the wave model of light works well for some phenomena, the particle model is necessary to explain others.
- Light can exhibit both wave-like and particle-like behaviour depending on the experiment performed. This is known as wave-particle duality.
- The wavelength of a photon determines its energy and frequency.
- Photons with longer wavelengths have lower energy and frequency, while photons with shorter wavelengths have higher energy and frequency.
- The wavelength of a photon can also affect its behaviour, such as its ability to penetrate materials or cause photochemical reactions.
Other properties of photons include:
- Photons have zero rest mass but have energy and momentum proportional to their frequency.
- Unlike other kinds of elementary particles, photons have no rest mass.
- Photons are electrically neutral, meaning they have no electric charge.
- Photons are stable particles that do not decay over time.
- Photons can interact with other particles, such as electrons, through processes such as absorption and emission.
- Photons can interact with other particles, such as electrons, through processes like absorption and emission.
- Photons always travel at the speed of light in a vacuum, regardless of their frequency or energy.
An electromagnetic wave carries electromagnetic radiation.
- An electromagnetic wave is formed as electromagnetic radiation propagates from a light source, travels through space and encounters different materials.
- Electromagnetic waves can be imagined as synchronised oscillations of electric and magnetic fields that propagate at the speed of light in a vacuum.
- Electromagnetic waves are similar to other types of waves in so far as they can be measured in terms of wavelength, frequency and amplitude.
- We can feel electromagnetic waves release their energy when sunlight warms our skin.
- Remember that electromagnetic radiation can be described either as an oscillating wave or as a stream of particles, called photons, which also travel in a wave-like pattern.
- The notion of waves is often used to describe phenomena such as refraction or reflection whilst the particle analogy is used when dealing with phenomena such as diffraction and interference.