A light-emitting diode (LED) is a semiconductor device that emits light when an electric current flows through it. Electroluminescence is the process where this happens: voltage applied to the semiconductor makes electrons flow across a junction, releasing energy as light.
Semiconductors and Light Emission
- Semiconductors, typically made from gallium nitride, are solid-state materials with unique properties that allow them to emit light at specific wavelengths, determining the perceived colour.
LED Colours
- LEDs typically emit one colour with a narrow range of wavelengths.
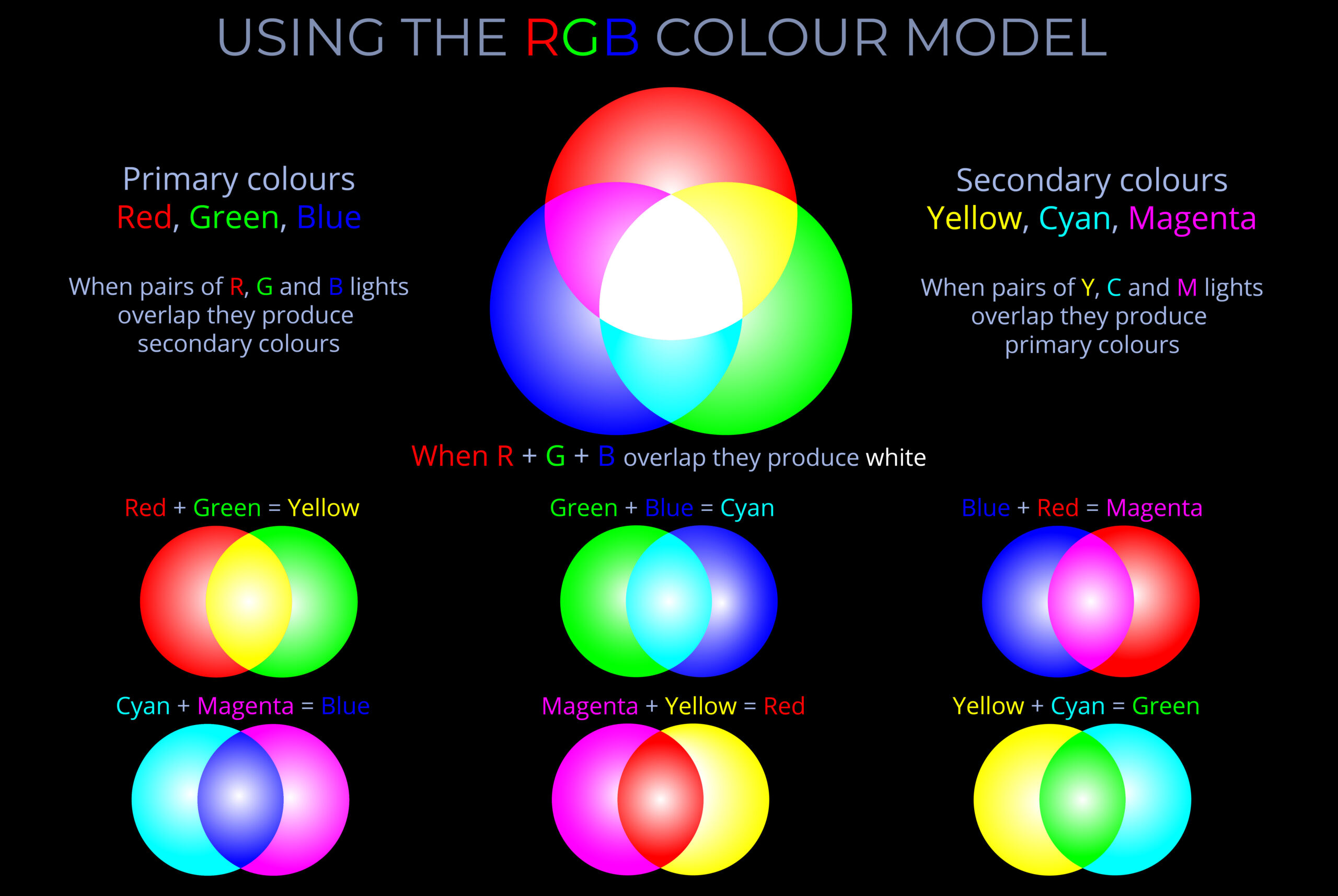
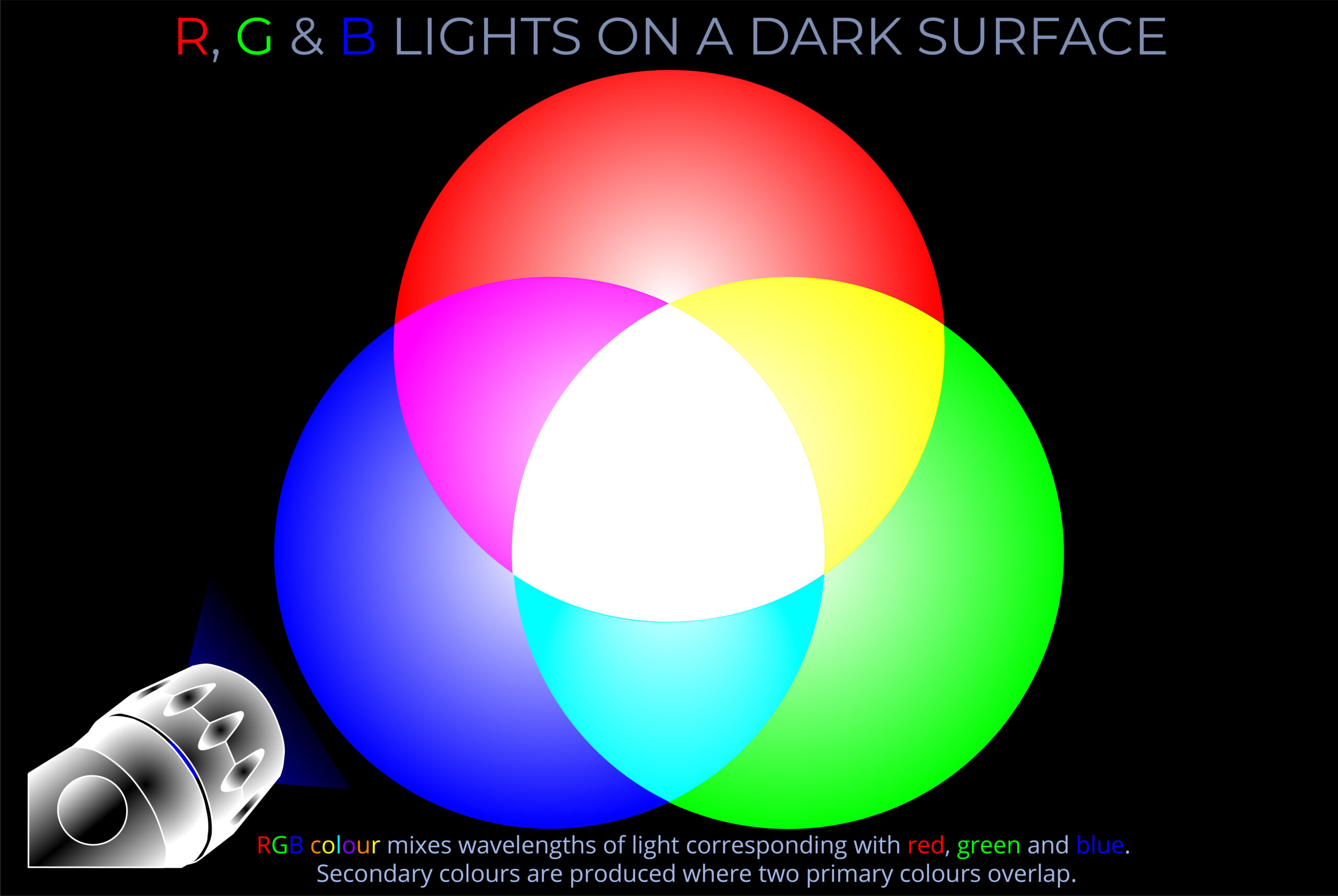
- Multicoloured LEDs combine three diodes emitting the RGB primary colours – red, green, and blue light.
- By adjusting the relative brightness of the primary colours, a vast array of colours can be created.
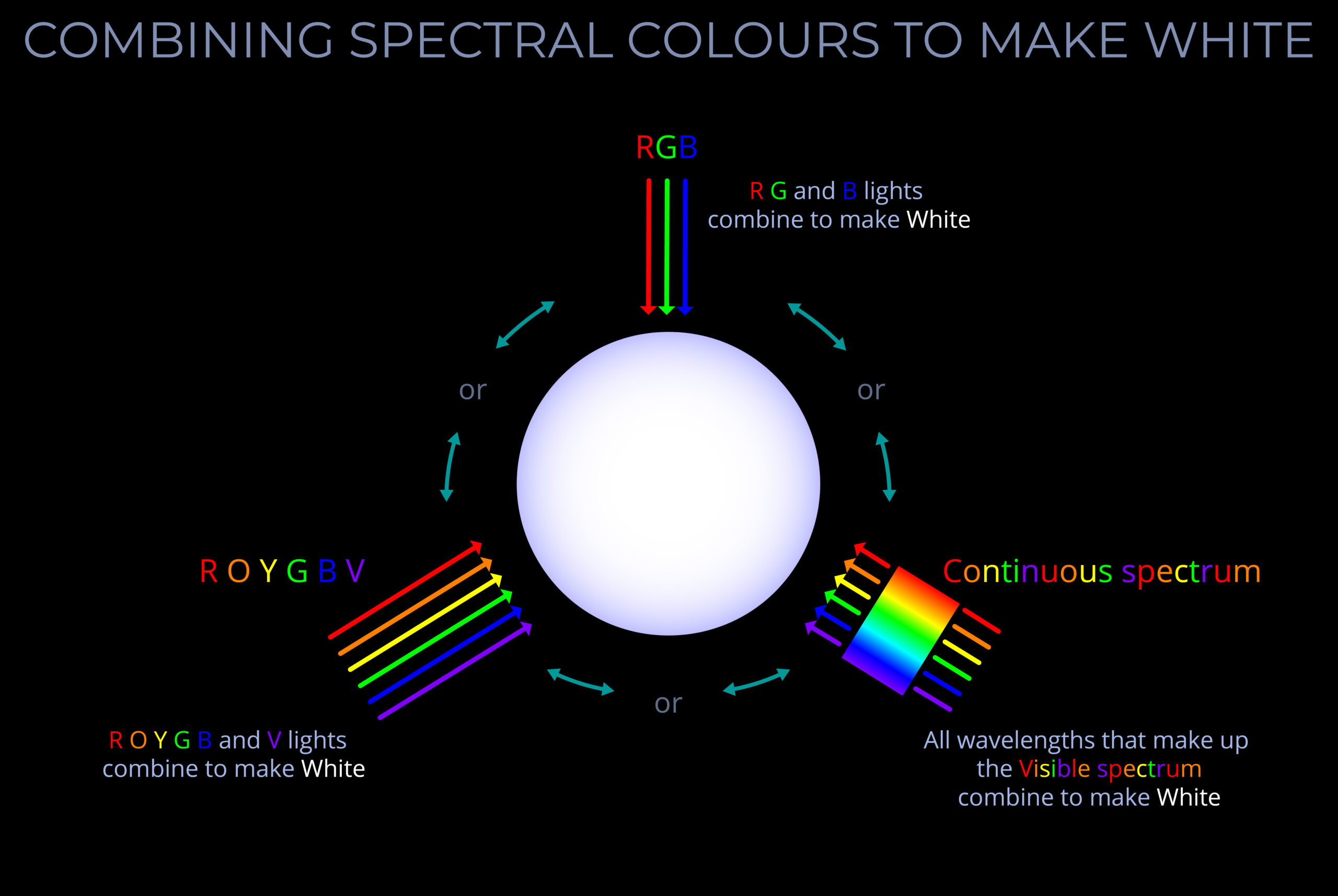
- Combining the three primary colours in equal proportions produces white light.
Role of Electrons in LEDs
- Applied Voltage: When connected to a power source, current flows through the LED exciting electrons: As electrons move across a junction within the semiconductor, they encounter empty energy levels called “holes”. Due to the attractive force between opposite charges, these electrons are drawn towards the holes and fill them. However, occupying a higher energy level places them in an unstable state.
- Energy Release: To reach a more stable state, each excited electron releases energy in the form of a photon (light packet). The energy of the emitted photon is equal to the energy difference between the higher energy level the electron occupied before and the lower energy level (the hole) it fills.
- Colour Control: The energy gap between the electron and the hole determines the photon’s energy, which translates to the perceived colour of the emitted light.
Holes in Semiconductors
- In semiconductor materials, “holes” represent the absence of an electron in a specific region where one could be.
- It’s not a physical hole or space but rather a way to understand the movement of electrons. These “holes” behave like positively charged particles because they represent a missing negative charge (electron).
| Light sources | Emission mechanism | Description | Examples |
|---|---|---|---|
| LIGHT-EMITTING PROCESS | |||
| Luminescence | Light emission due to the excitation of electrons in a material. | Electrons within a material gain energy and then release light as they return to a lower energy state. | Bioelectroluminescence Electroluminescence Photoluminescence - Fluorescence - Phosphorescence Sonoluminescence Thermoluminescence |
| Blackbody radiation (Type of thermal radiation) | Electromagnetic radiation (including visible light) emitted by any object with a temperature above absolute zero. | Electromagnetic radiation (including visible light) emitted by any object with a temperature above absolute zero. | All objects above temperature of absolute zero. |
| Chemiluminescence | Light from natural and artificial chemical reactions. | Light from natural and artificial chemical reactions. | Bioluminescence Chemiluminescent reactions: - Luminol reactions - Ruthenium chemiluminescence |
| Nuclear reaction | Light emission as a byproduct of nuclear reactions (fusion or fission). | Light emitted as a byproduct of nuclear reactions. | Nuclear reactors Stars undergoing fusion |
| Thermal radiation | Light emission due to the thermal excitation of atoms and molecules at high temperatures. | Light emission due to the thermal excitation of atoms and molecules. | Sun Stars Incandescent light bulbs |
| Triboluminescence | Light emission due to mechanical stress applied to a material. | Light emission due to the mechanical stress applied to a material, causing the movement of electric charges and subsequent light emission. | Sugar crystals cracking Adhesive tape peeling Quartz crystals fracturing. |
| Natural light source | |||
| Fireflies Deep-sea creatures Glowing mushrooms | Bioluminescence | Light emission from biological organisms. | Involves the luciferase enzyme. |
| Sun Stars | Nuclear Fusion | Light emission as a byproduct of nuclear fusion reactions in stars. | Electromagnetic spectrum (visible light, infrared, ultraviolet). |
| Fire Candles | Thermal radiation | Light emission due to the thermal excitation of atoms and molecules during the combustion of a fuel source. | Burning of a fuel source, releasing heat and light. |
| Artificial light source | |||
| Fluorescent lights Highlighters Safety vests | Chemiluminescence | Light emission from chemical reactions. | Fluorescence (absorption and re-emission of light). |
| Glow sticks Emergency signs | Chemiluminescence | Light emission due to phosphorescence - a type of chemiluminescence. | A type of chemiluminescence where light emission is delayed after the initial excitation. |
| Glow sticks Light sticks | Chemiluminescence | Chemiluminescence | Light emission from a chemical reaction that does not involve combustion. |
| Tungsten light bulbs Toasters | Thermal radiation | Heated filament radiates light and heat. | Light emission from a hot filament. |
| Fluorescent lamps LED lights | Electroluminescence | Excitation of atoms by electric current. | Light emission when electric current excites atoms in a material. |
| Neon signs | Electrical Discharge | Discharge of electricity through gas. | Light emission when electricity flows through a gas. |
| Sugar crystals cracking Pressure-sensitive adhesives | Triboluminescence | Light emission from friction or pressure. | Light emission due to mechanical forces. |
| Fluorescent paint Highlighters Safety vests | Photoluminescence | Absorption and subsequent re-emission of light at a lower energy. | Absorption and re-emission of light. |
Light Sources: Mechanism, examples, and everyday applications
Footnote: Cerenkov radiation and Synchrotron radiation are not included in the table because they are not conventionally classified as light sources.
- A light-emitting diode (LED) is a semiconductor device that emits light when an electric current flows through it. Electroluminescence is the process where this happens: voltage applied to the semiconductor makes electrons flow across a junction, releasing energy as light.
- Semiconductors, typically made from gallium nitride, are solid-state materials with unique properties that allow them to emit light at specific wavelengths, determining the perceived colour.
- LEDs typically emit one colour with a narrow range of wavelengths.
- Multicoloured LEDs combine three diodes emitting the RGB primary colours – red, green, and blue light.
- By adjusting the relative brightness of the primary colours, a vast array of colours can be created.
- Combining the three primary colours in equal proportions produces white light.