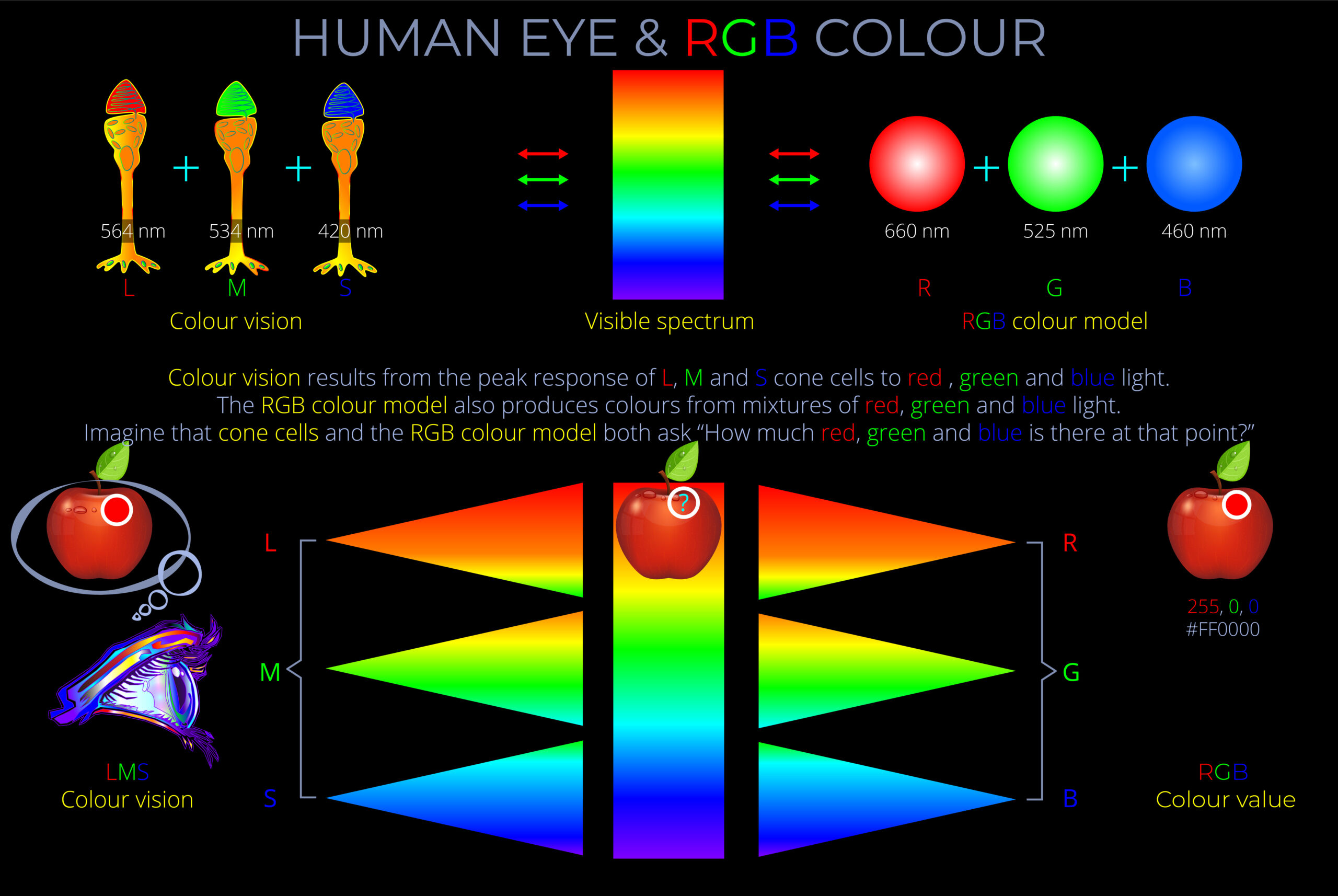
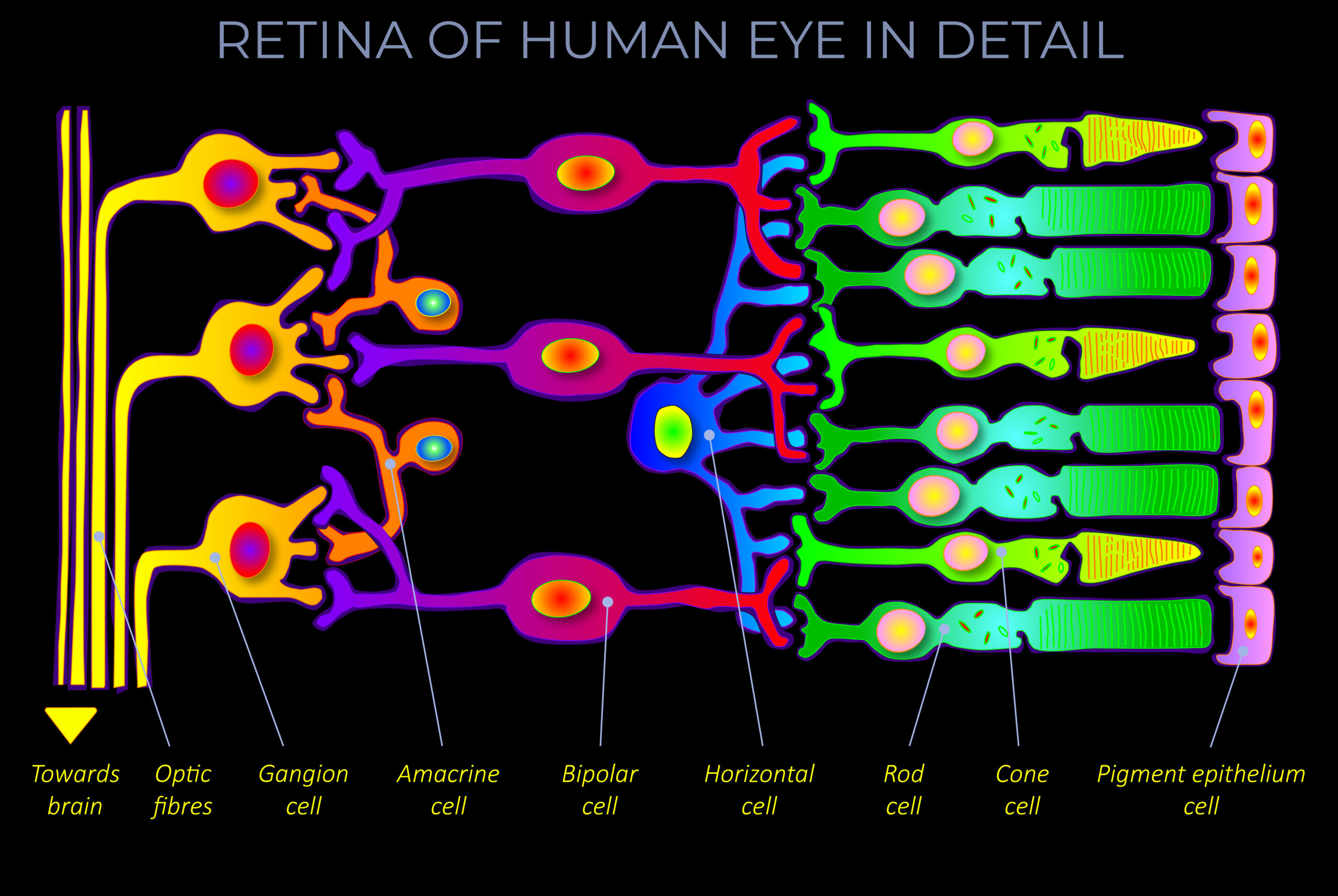
The apparent colour of an object is determined as electrons absorb some wavelengths of light and reflect others. The colour an observer sees corresponds with the reflected wavelengths.
- In terms of everyday experience, three key factors affect the colour of an object:
- The light source and what happens to the light on its journey towards an object.
- What happens when light strikes a material or medium.
- Factors related to an observer that affect the way they perceive things.
At an atomic level, things appear to have colour because chromophores within molecules absorb specific wavelengths of light while reflecting others.
- A chromophore is the part of a molecule that is responsible for the absorption of some wavelengths of light and not others.
- Chromophores are the specific groups of atoms within a larger molecule that determine its colour.
- It is the structure of chromophores that determines which wavelengths of light they absorb and so also the wavelengths that are reflected.
- The apparent colour of a surface or opaque object corresponds with the wavelengths of light that are not absorbed during their interaction with the chromophore.
- For example, a chromophore found in carrots (called beta-carotene) absorbs light in the blue region of the spectrum, while other wavelengths (corresponding with red and yellow) are reflected, giving the vegetable its characteristic orange colour.
- Chromophores are not only found in natural compounds but also in man-made materials such as dyes and pigments. The wide variety of types of chromophores and their molecular structures allows for the vast range of colours seen in these substances.
How it Works
- To understand this process remember that molecules are made up of atoms with bonds between them. The bonds result from the atoms sharing electrons.
- When atoms share electrons in a series of alternating single and double bonds they produce a structure, known as a conjugated system.
- It is this structure that is integral to how chromophores function.
- The way in which electrons share molecules depends on the type of atoms involved, the number of electrons shared, and the way they are shared.
- So for example, In a typical chemical substance that consists of molecules of the same composition and structure, the chromophore is usually identified with a region which has a conjugated system.
- When this region encounters a photon of light, the photon’s energy is transferred to an electron in the conjugated system causing it to move to a higher energy level, or “orbit”.
- As an electron moves to a higher orbit, it goes from a stable, lower-energy state (the ground state) to a less stable, higher-energy state (an excited state).
- Various factors in a chromophore’s structure determine which wavelengths it will absorb and in simple terms, two rules apply:
- If the energy of the photon matches the energy difference between the electron’s current energy level and a higher orbital, the electron can move to that higher energy level.
- The longer the conjugated system with a chromophore the longer the wavelengths absorbed.
- An electron in an excited state is less stable and will eventually return to the ground state. As it does so, it emits energy in the form of visible light (or heat).
- However, while it is true that electrons returning to their ground state from an excited state emit photons, the process that determines the colour we perceive in objects such as pigments is mainly governed by the absorption of light, rather than the re-emission of light because the emitted light is usually less intense than the reflected light
- So it is the structure of a chromophore, particularly the size and arrangement of its conjugated system, that determines the energy difference between the ground and excited states of its electrons. This in turn affects which wavelengths of light are absorbed and which are reflected, defining the colour that we perceive from the molecule.
Chromophores & Molecular Orbitals
- The chromophore is the part of a molecule where there is an energy difference between two different molecular orbitals.
- A molecular orbital refers to the position and wave-like behaviour of an electron as it moves around an atom’s nucleus.
- If the energy difference of a chromophore falls within the range of the visible spectrum (2 to 2.75 electron volts) then it will produce colour.
- The apparent colour of an object is determined as electrons absorb some wavelengths of light and reflect others. The colour an observer sees corresponds with the reflected wavelengths.
- In terms of everyday experience, three key factors affect the colour of an object:
- The light source and what happens to the light on its journey towards an object.
- What happens when light strikes a material or medium.
- Factors related to an observer that affect the way they perceive things.
- At an atomic level, things appear to have colour because chromophores within molecules absorb specific wavelengths of light while reflecting others.