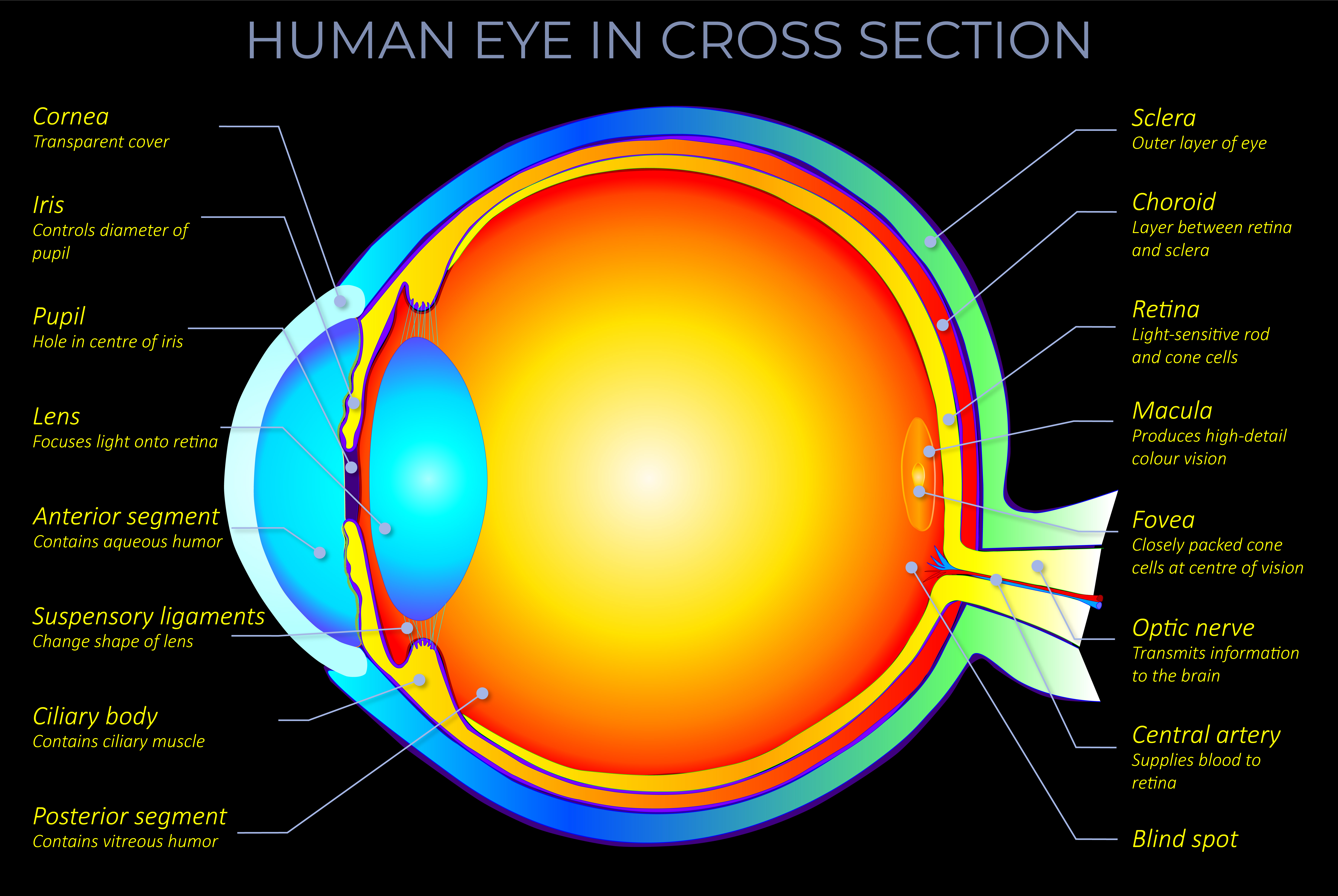
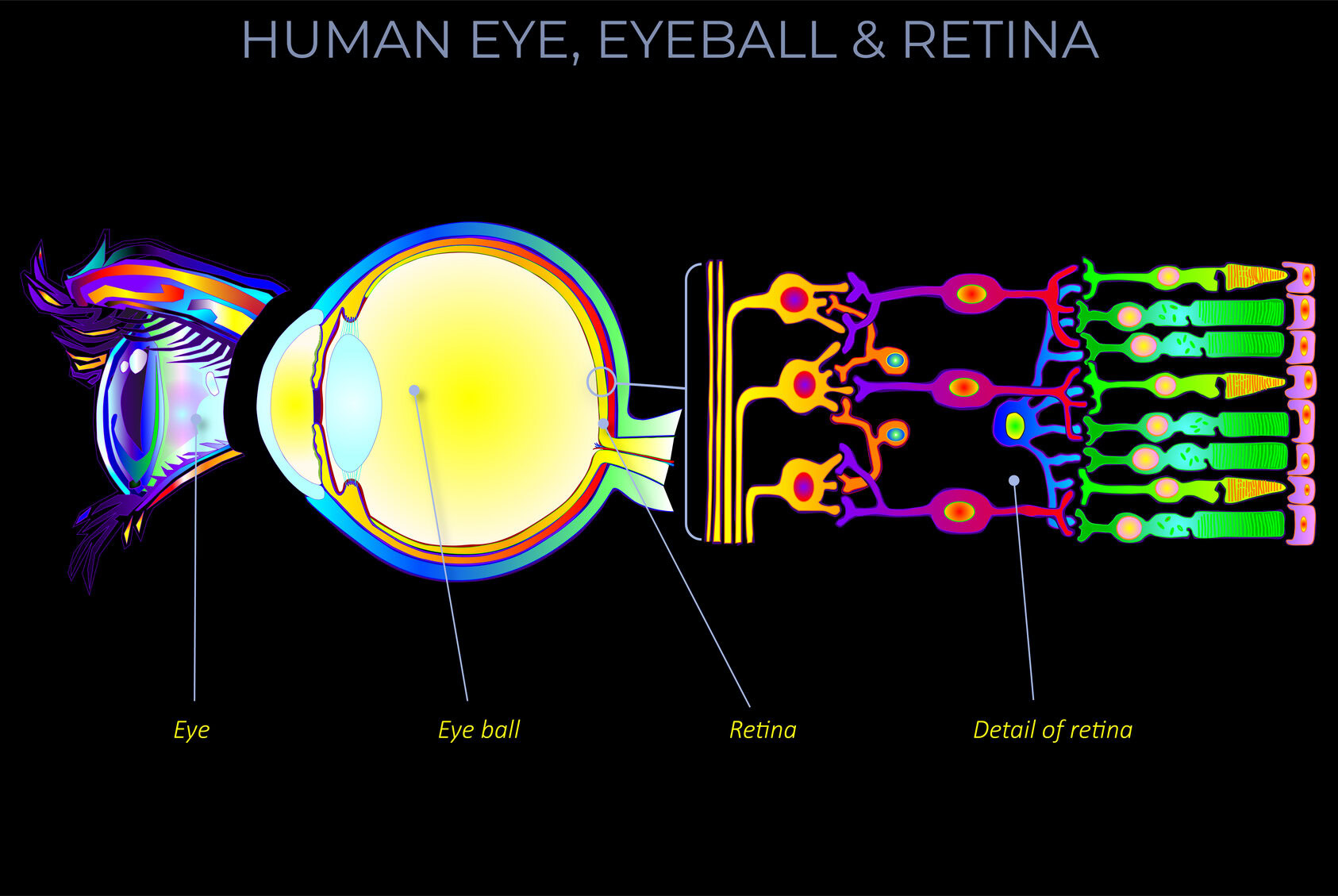
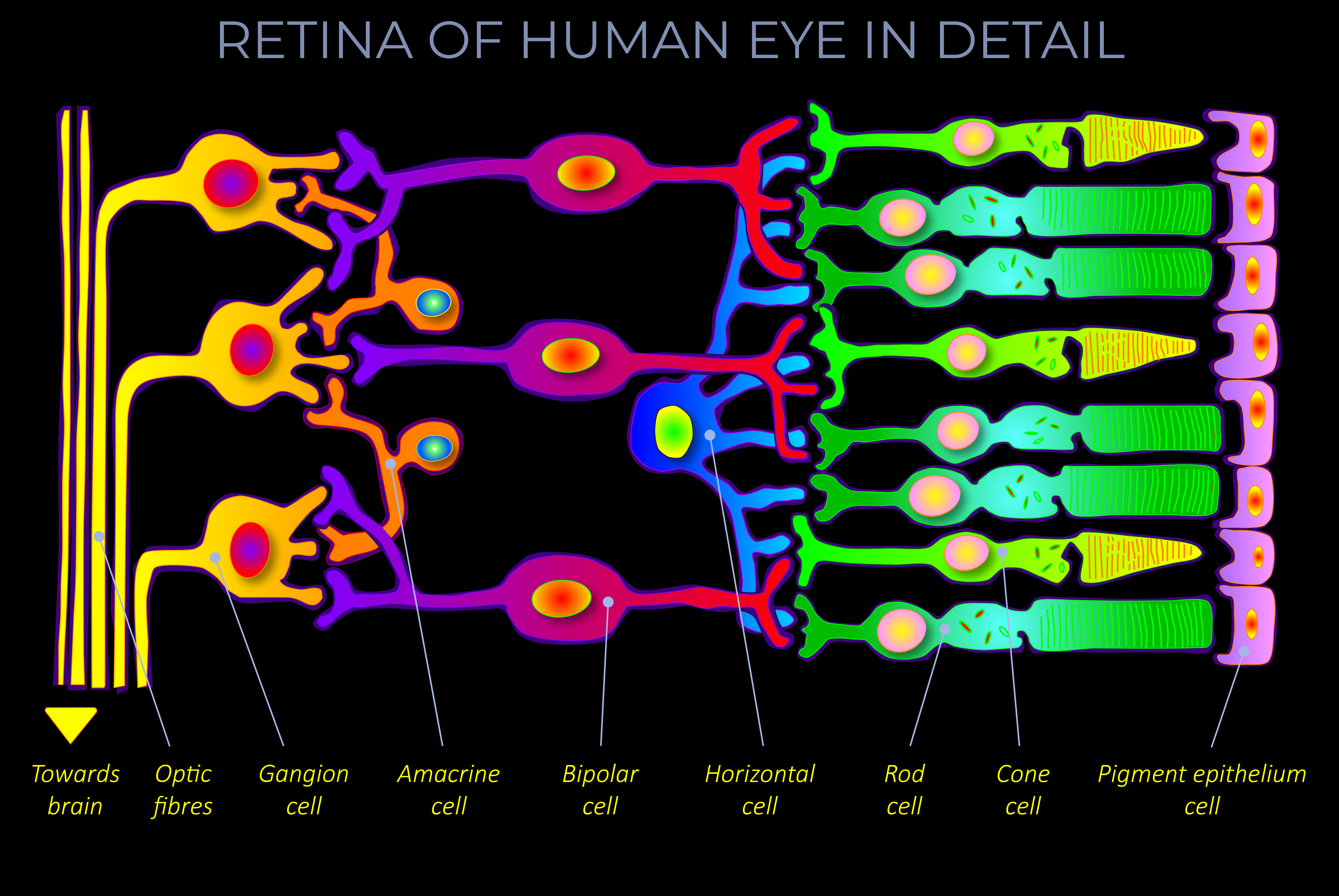
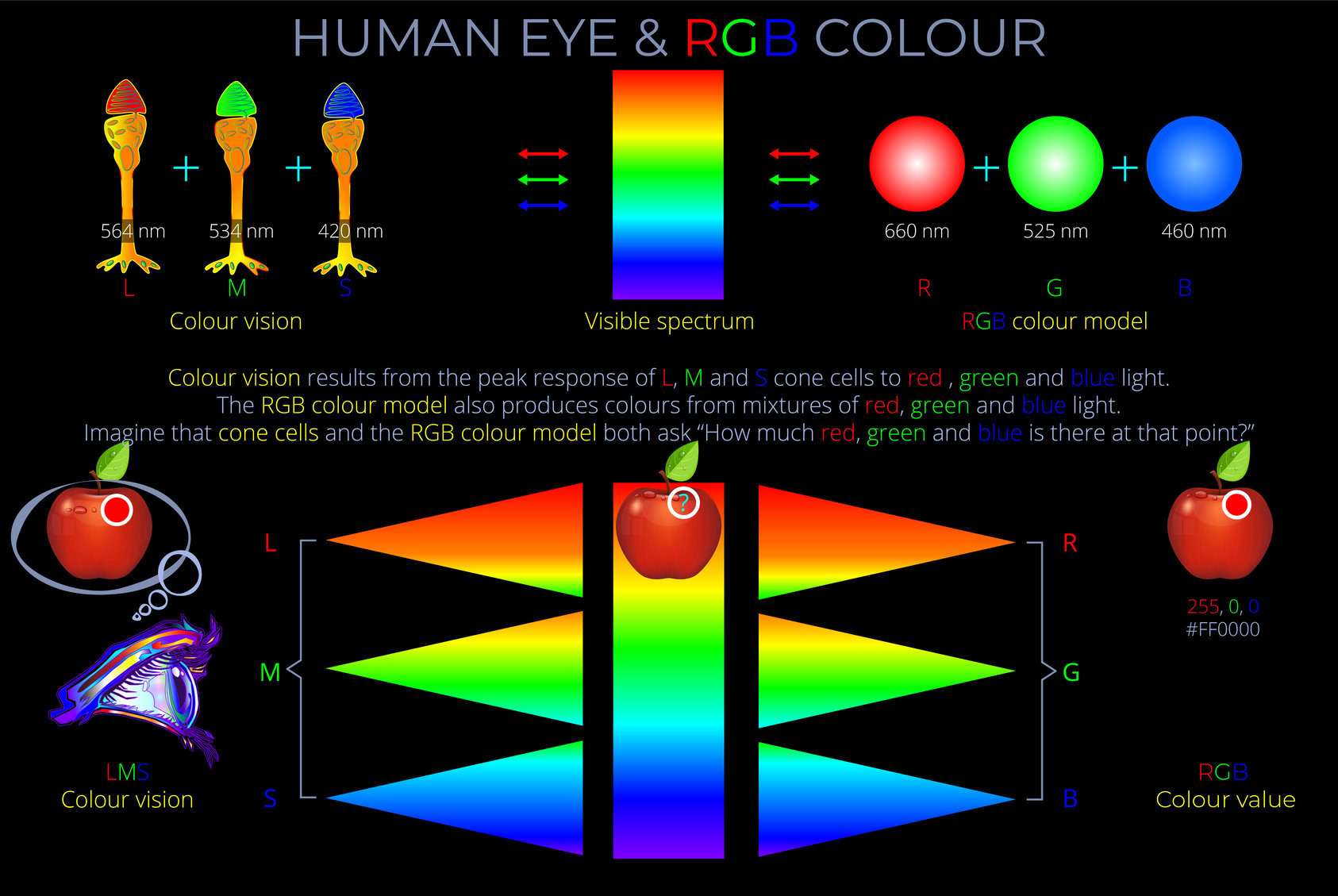
Kinetic energy is the energy an object has because of its motion.
- Planets, cars, people and atoms all have kinetic energy due to their motion.
- When a force is applied to an object, its kinetic energy can change.
- Kinetic energy is the energy of motion, while potential energy is the energy of position or state.
- Most interactions between objects involve forces and can transfer energy.
A laser is a light source that can create a narrow and intense beam of electromagnetic radiation. Unlike a flashlight, which has a bulb that emits light in all directions, a laser beam focuses its light into a concentrated stream of photons. LASER stands for Light Amplification by Stimulated Emission of Radiation.
- Light waves are made up of tiny packets of energy called photons.
- Normal light emission happens when atoms or molecules release photons when they transition from higher energy states to lower ones. These emitted photons have random directions and energies, creating a diffuse light.
- The concept that makes lasers unique is stimulated emission. This occurs when an incoming photon interacts with an excited atom in the laser material. The photon’s energy triggers the excited atom in the material to emit a new photon with identical characteristics. The new photon has the same wavelength and so colour, phase and direction as the original.
- Laser material refers to the medium that is used to generate the laser light.
- This phenomenon creates a cascade effect. The newly emitted photon can itself stimulate another excited atom, leading to two identical photons travelling in the same direction. This process repeats, rapidly amplifying the initial light within the laser cavity.
- The cavity comprises two mirrors strategically positioned at the opposite ends of the laser material. One mirror is fully reflective, while the other partially reflects.
- As the amplified light bounces between the mirrors, it continues to stimulate more emissions, resulting in an intense beam of identical photons. The partially reflective mirror allows a portion of this intense light to escape as the laser beam, while the rest continues to contribute to the amplification within the cavity.
Lateral geniculate nucleus
The lateral geniculate nucleus is a relay centre on the visual pathway from the eyeball to the brain. It receives sensory input from the retina via the axons of ganglion cells.
The thalamus which houses the lateral geniculate nucleus is a small structure within the brain, located just above the brain stem between the cerebral cortex and the midbrain with extensive nerve connections to both.
The lateral geniculate nucleus is the central connection for the optic nerve to the occipital lobe of the brain, particularly the primary visual cortex.
Both the left and right hemispheres of the brain have a lateral geniculate nucleus.
There are three major cell types in the lateral geniculate nucleus which connect to three distinct types of ganglion cells:
- P ganglion cells send axons to the parvocellular layer of the lateral geniculate nucleus.
- M ganglion cells send axons to the magnocellular layer.
- K ganglion cells send axons to a koniocellular layer.
The lateral geniculate nucleus specialises in calculations based on the information it receives from both the eyes and from the brain. Calculations include resolving temporal and spatial correlations between different inputs. This means that things can be organised in terms of the sequence of events over time and the spatial relationship of things within the overall field of view.
Some of the correlations deal with signals received from one eye but not the other. Some deal with the left and right semi-fields of view captured by both eyes. As a result, they help to produce a three-dimensional representation of the field of view of an observer.
- The outputs of the lateral geniculate nucleus serve several functions. Some are directed towards the eyes, others are directed towards the brain.
- A signal is provided to control the vergence of the two eyes so they converge at the principal plane of interest in object-space at any particular moment.
- Computations within the lateral geniculate nucleus determine the position of every major element in object-space relative to the observer. The motion of the eyes enables a larger stereoscopic mapping of the visual field to be achieved.
- A tag is provided for each major element in the central field of view of object-space. The accumulated tags are attached to the features in the merged visual fields and are forwarded to the primary visual cortex.
- Another tag is provided for each major element in the visual field describing the velocity of the major elements based on changes in position over time. The velocity tags (particularly those associated with the peripheral field of view) are also used to determine the direction the organism is moving relative to object-space.
The lateral geniculate nucleus (LGN) is a relay centre in the visual pathway from the eye to the brain. It receives signals from the retina via the axons of ganglion cells. The thalamus, a part of the brain located near the brainstem, houses the LGN.
- The thalamus which houses the lateral geniculate nucleus is a small structure within the brain, located just above the brain stem between the cerebral cortex and the midbrain and has extensive nerve connections to both.
- The LGN specializes in processing visual information from both eyes. It resolves relationships between different visual inputs, helping us understand the sequence of events and the location of objects in our field of view.
- Some of this processing involves signals from one eye, while others deal with information from both eyes to create a three-dimensional perception of the world. The LGN acts as a central connection for the optic nerve to the primary visual cortex in the occipital lobe. Both the left and right hemispheres of the brain have a lateral geniculate nucleus.
- There are three major cell types in the LGN, each connecting to different types of ganglion cells and playing specific roles in vision:
- P cells: Process information about colour and fine detail.
- M cells: Respond to motion.
- K cells: Involved in low-resolution processing.
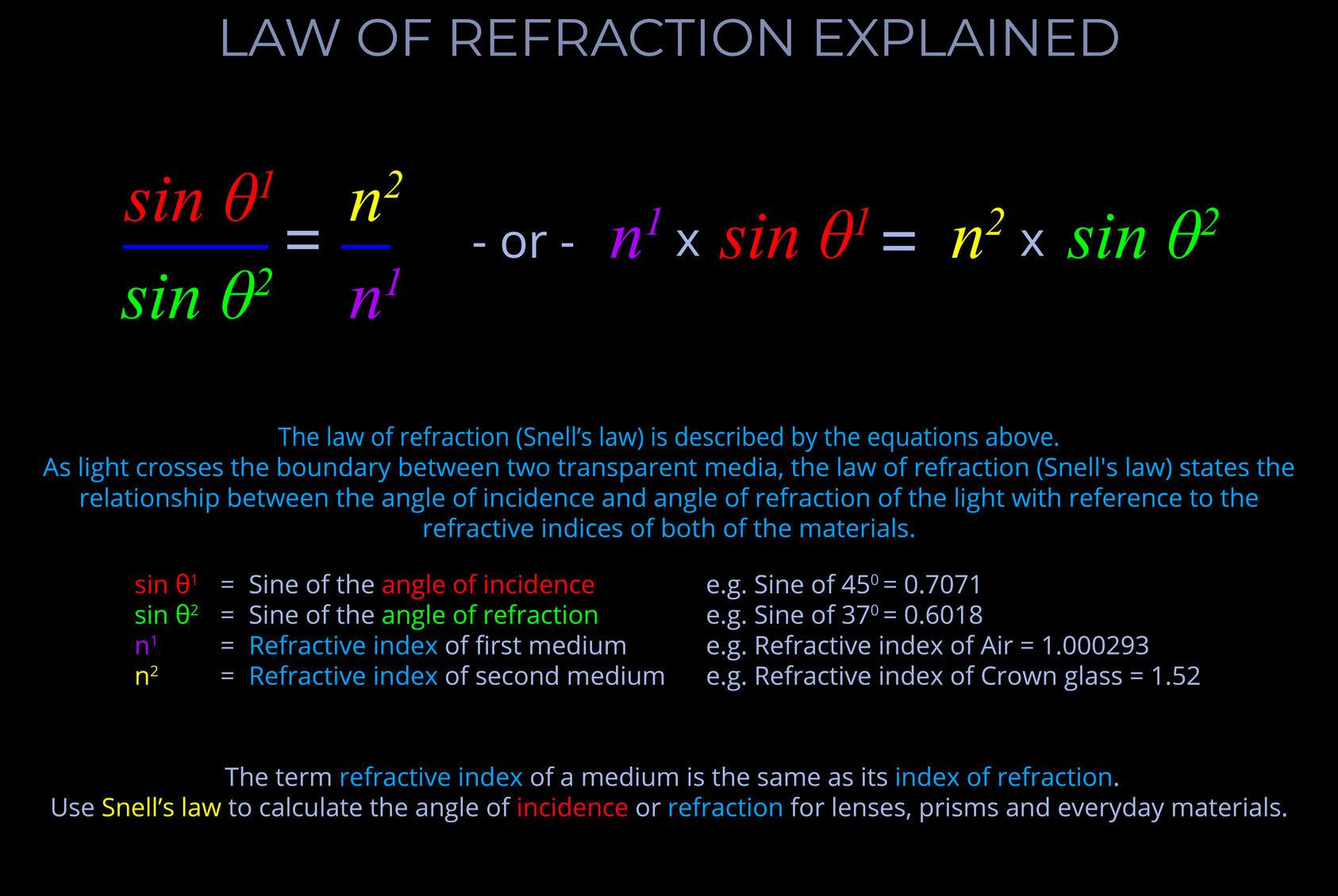
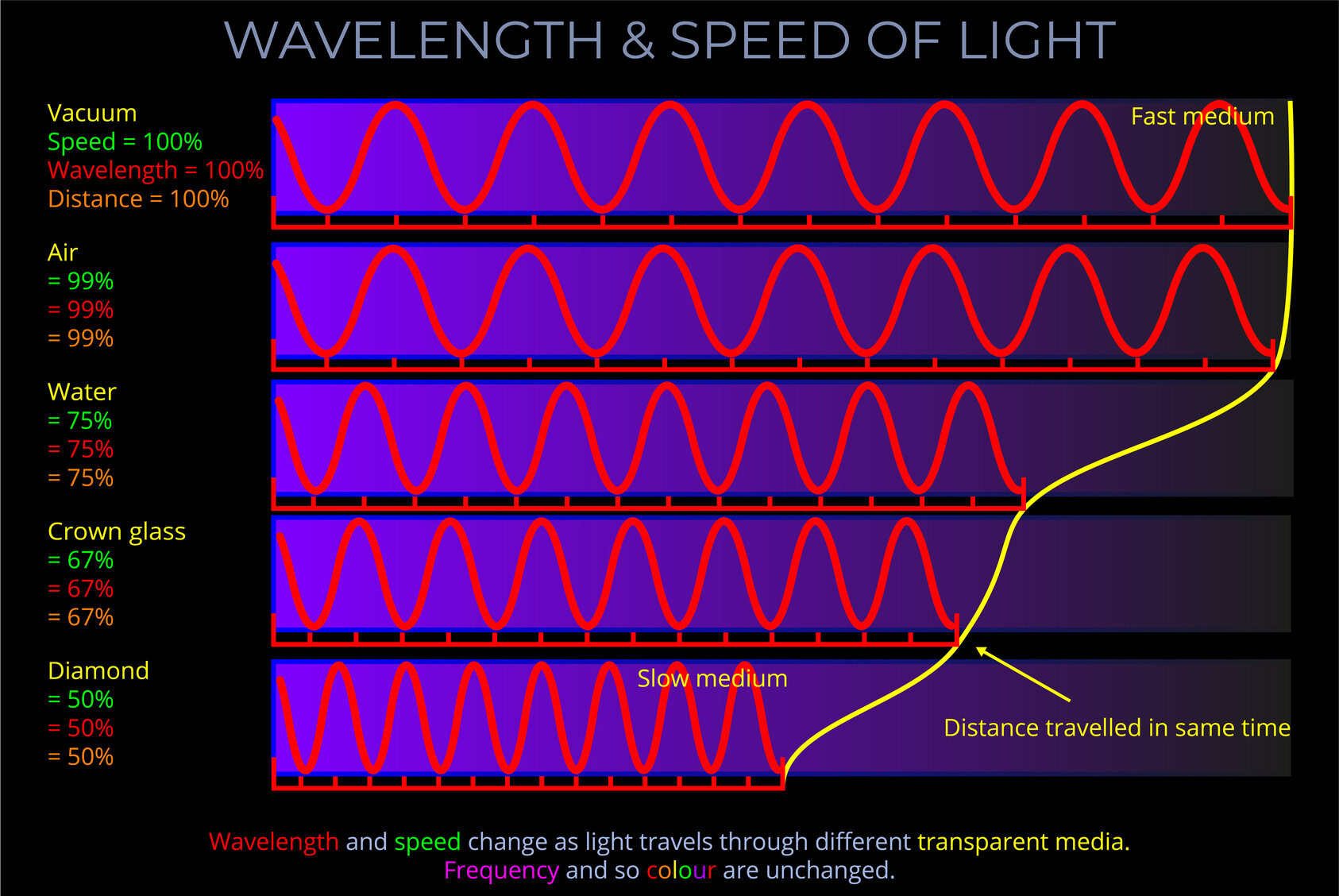
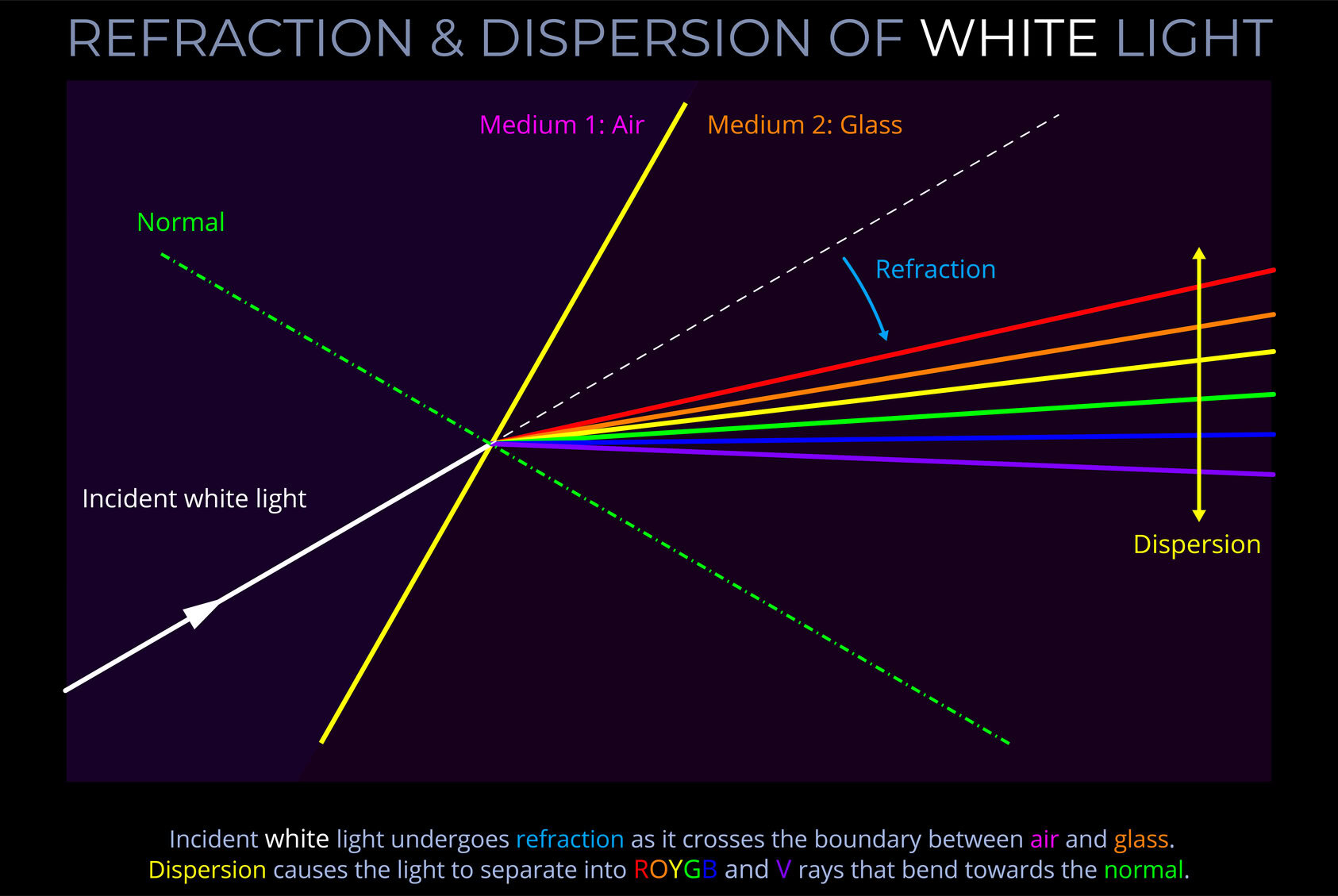
As light crosses the boundary between two transparent media, the law of refraction (Snell’s law) states the relationship between the angle of incidence and angle of refraction of the light with reference to the refractive indices of both media as follows:
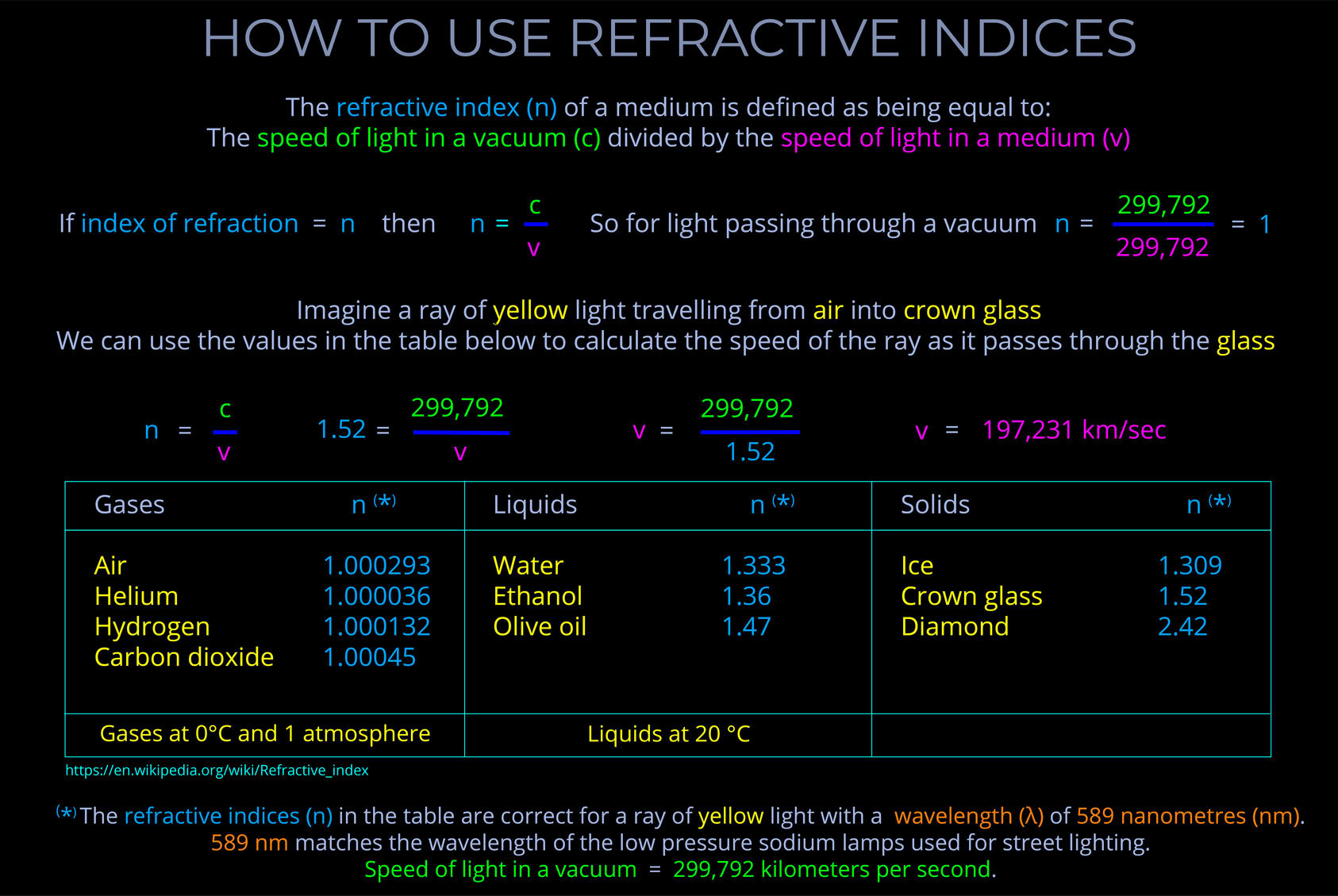
When electromagnetic radiation (light) of a specific frequency crosses the interface of any given pair of media, the ratio of the sines of the angles of incidence and the sines of the angles of refraction is a constant in every case.
- Snell’s law deals with the fact that for an incident ray approaching the boundary of two media, the sine of the angle of incidence multiplied by the index of refraction of the first medium is equal to the sine of the angle of refraction multiplied by the index of refraction of the second medium.
- Snell’s law deals with the fact that the sine of the angle of incidence to the sine of the angle of refraction is constant when a light ray passes across the boundary from one medium to another.
- Snell’s law can be used to calculate the angle of incidence or refraction associated with the use of lenses, prisms and other everyday materials.
- When using Snell’s law:
- The angles of incidence and refraction are measured between the direction of a ray of light and the normal – where the normal is an imaginary line drawn on a ray diagram perpendicular to, so at a right angle to (900), to the boundary between two media.
- The wavelength of the incident light is accounted for.
- The refractive indices used are selected for the pair of media concerned.
- The speed of light is expressed in metres per second (m/s).
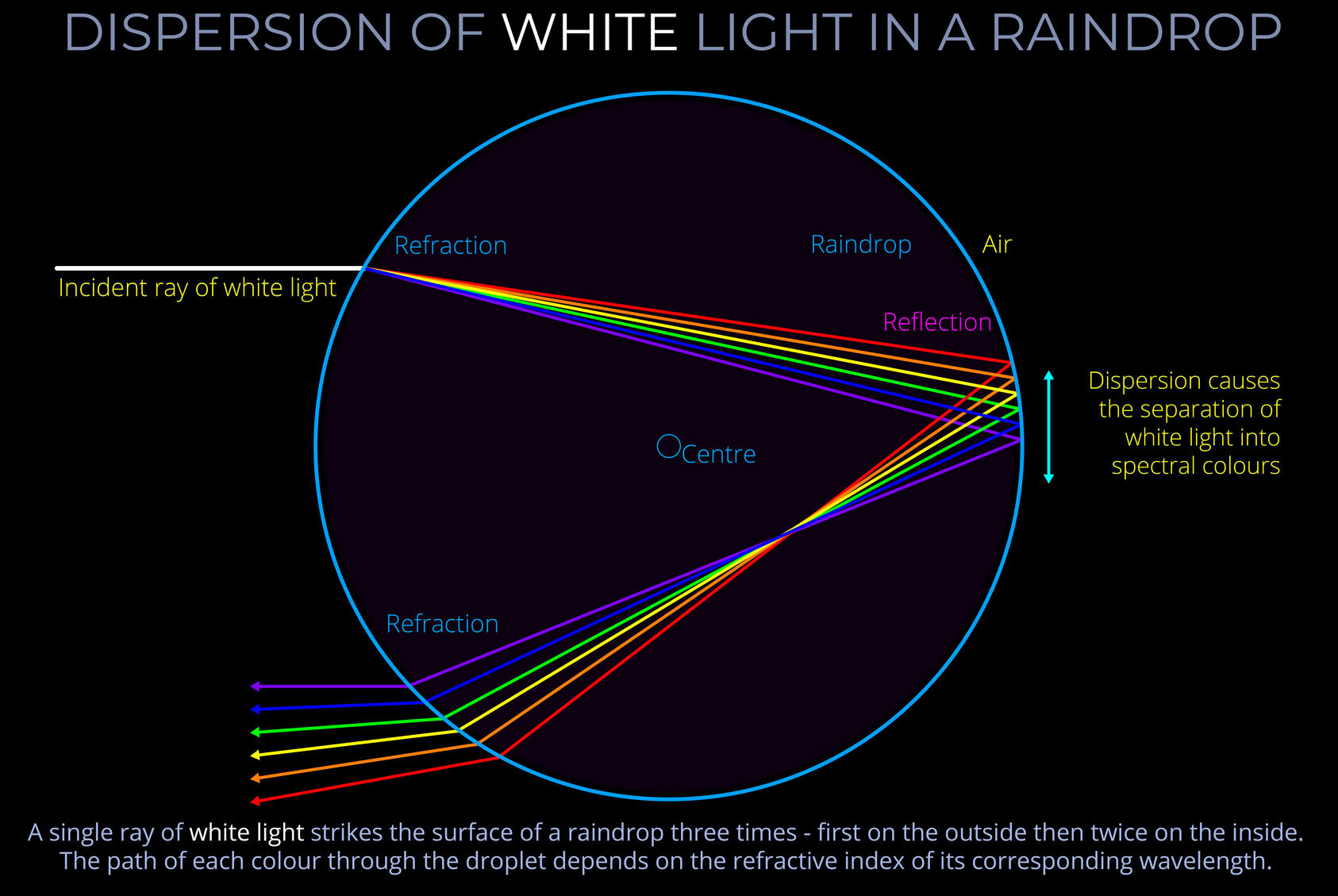
The path of light through a raindrop is a key factor in determining whether it will direct light towards an observer and contribute to their perception of a rainbow. This can be broken down as follows:
- The impact parameter is a measure of the direction from which rays of incident light approach a raindrop and the point at which they strike the surface.
- When using a ray-tracing diagram to map the path of rays through a raindrop, an impact parameter scale is used to select which incident rays are of interest.
- An impact parameter scale is aligned with parallel incident rays and divides the relevant part of the surface of a droplet into equal parts.
- Using a scale with steps between zero and one, 0 is aligned with the ray that passes through the centre of a droplet and 1 with the ray that grazes the surface without refraction or reflection.
Remember that:
- Primary rainbows form when incident light strikes raindrops above their horizontal axis reflecting once off the inside before exiting towards an observer.
- Incident light that strikes raindrops below their horizontal axis and reflects once on the inside before exiting, directs light upwards away from an observer.
- Secondary rainbows form when incident light strikes raindrops below their horizontal axis reflecting twice off the inside before exiting downwards.
- The Law of reflection deals with the angles of incidence and reflection when light strikes and bounces back off a surface and can be used for calculations relating to the curved surfaces of a raindrop.
- Remember that the law of reflection states that the angle of incidence always equals the angle of reflection for a mirror-like (specular) surface.
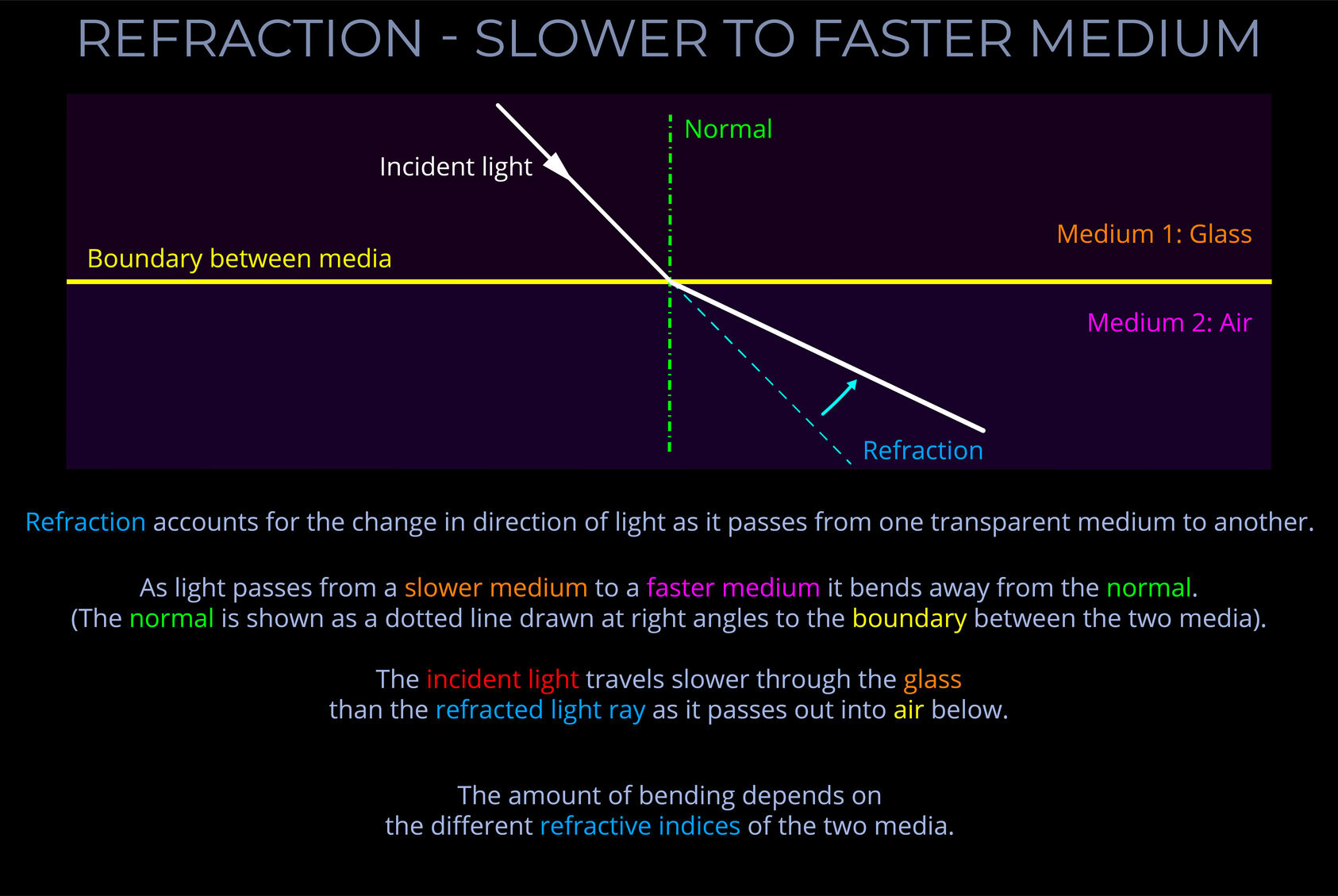
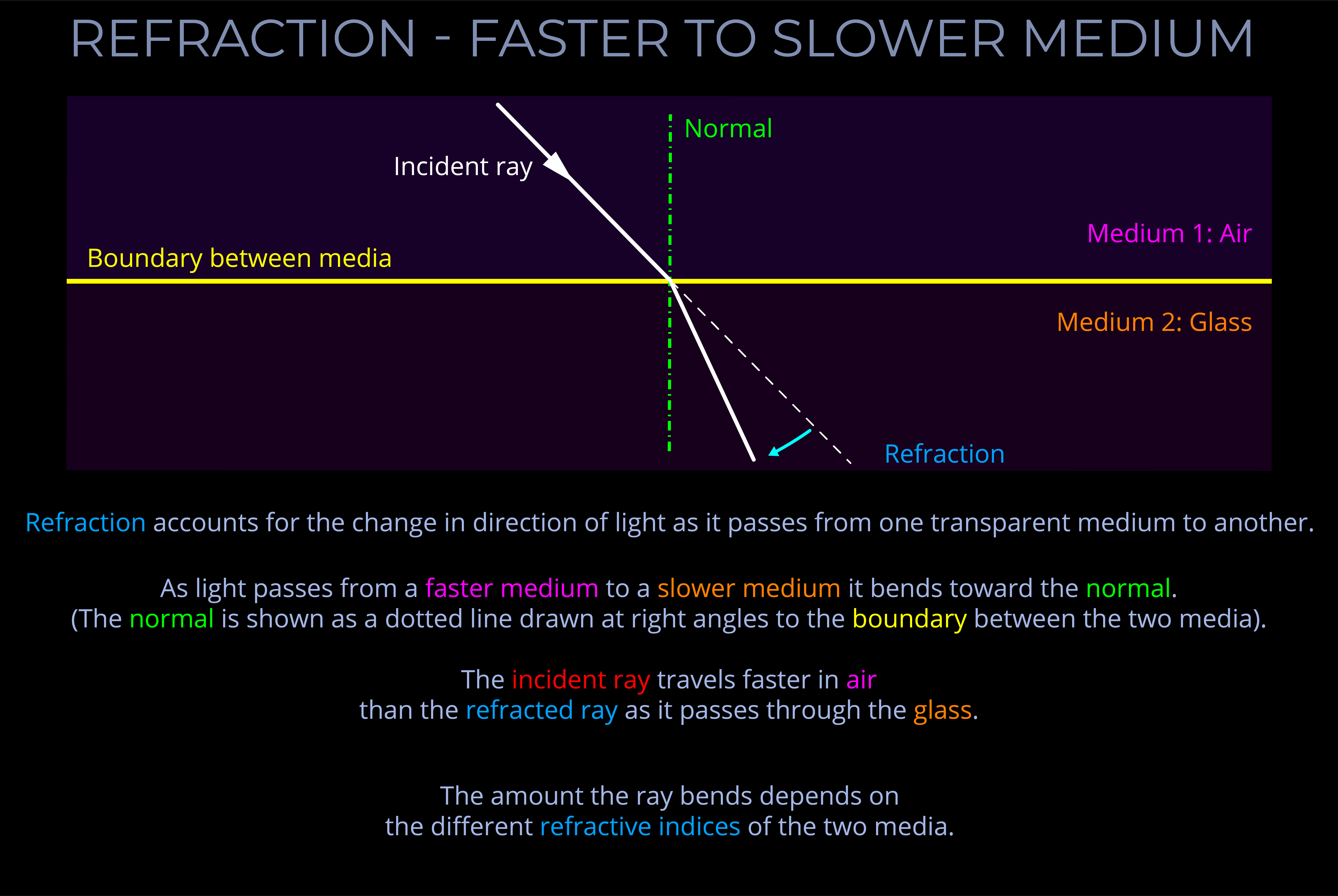
- The Law of Refraction (Snell’s law) deals with the changes in the speed and direction of incident light as it crosses the boundaries between air and a raindrop and then between a raindrop and the surrounding air.
- An equation can be derived from Snell’s law that deals with the relationship between the angle of incidence and the angle of refraction of light with reference to the refractive indices of both media.
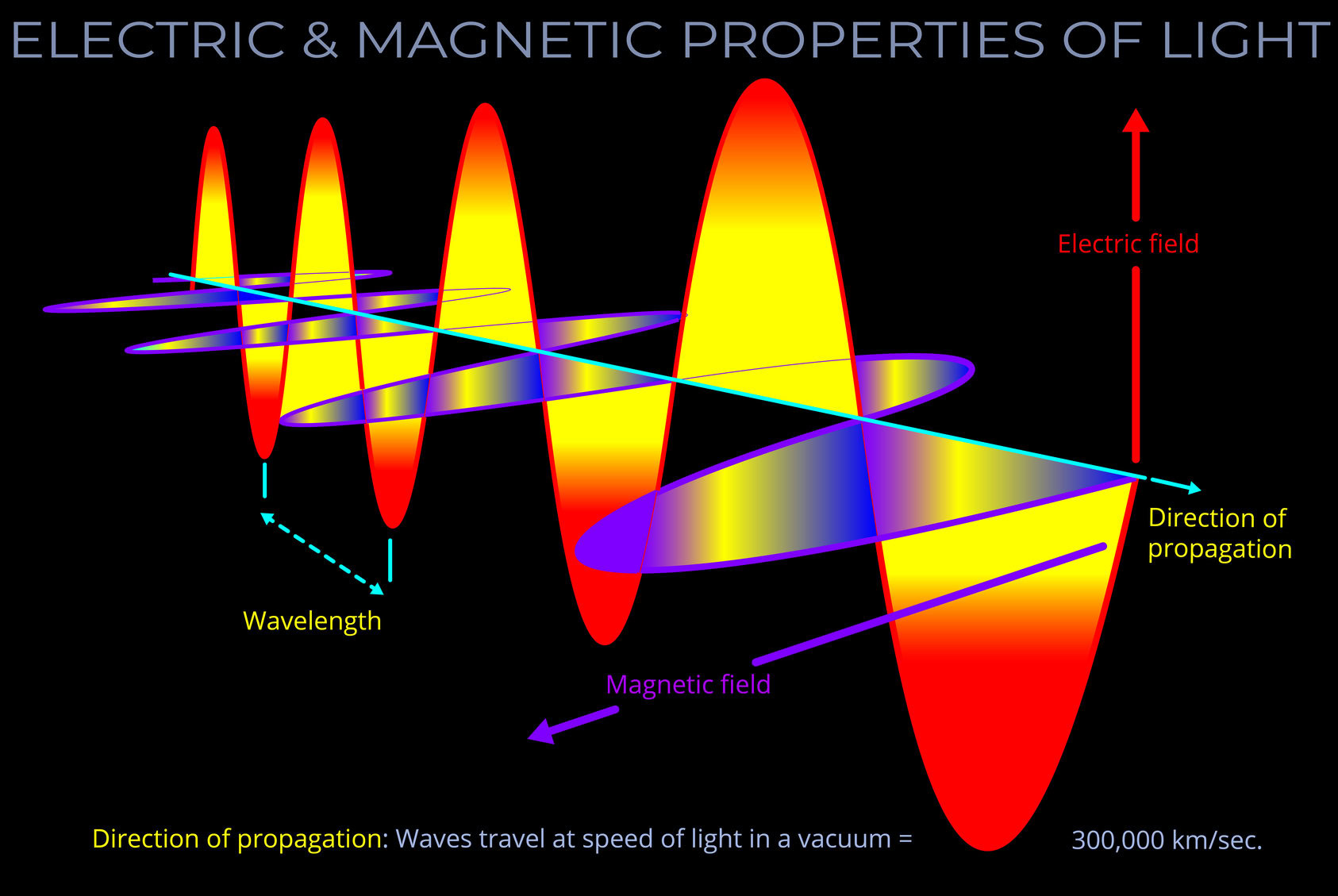
Light is electromagnetic radiation (radiant energy), which, detached from its source, is transported by electromagnetic waves (or their quanta, photons) and propagates through space. Even if humans had never evolved, stars would have emitted electromagnetic radiation since the first galaxies formed over 13 billion years ago.
- Simply stated, light is energy. Light is the way energy travels through space.
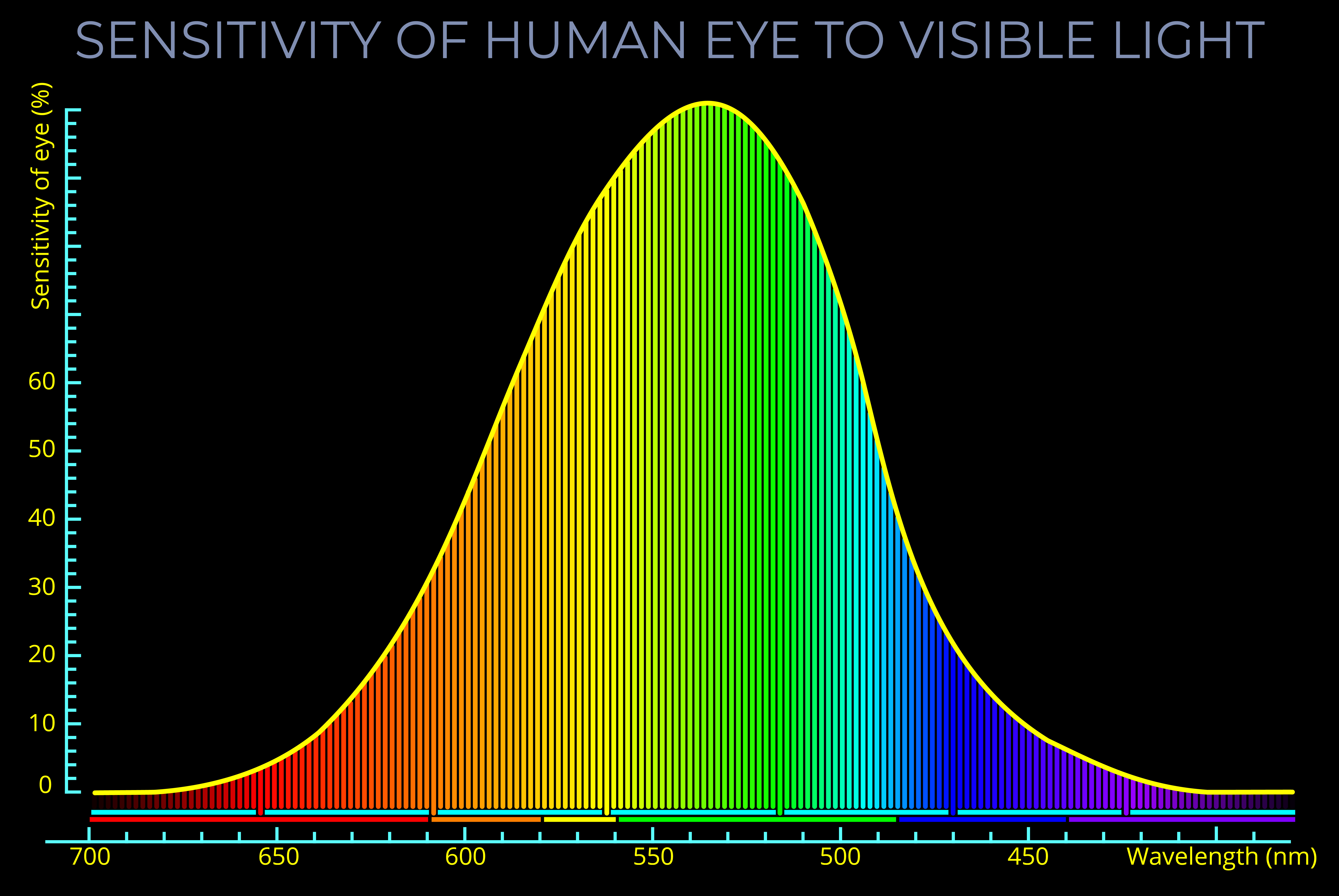
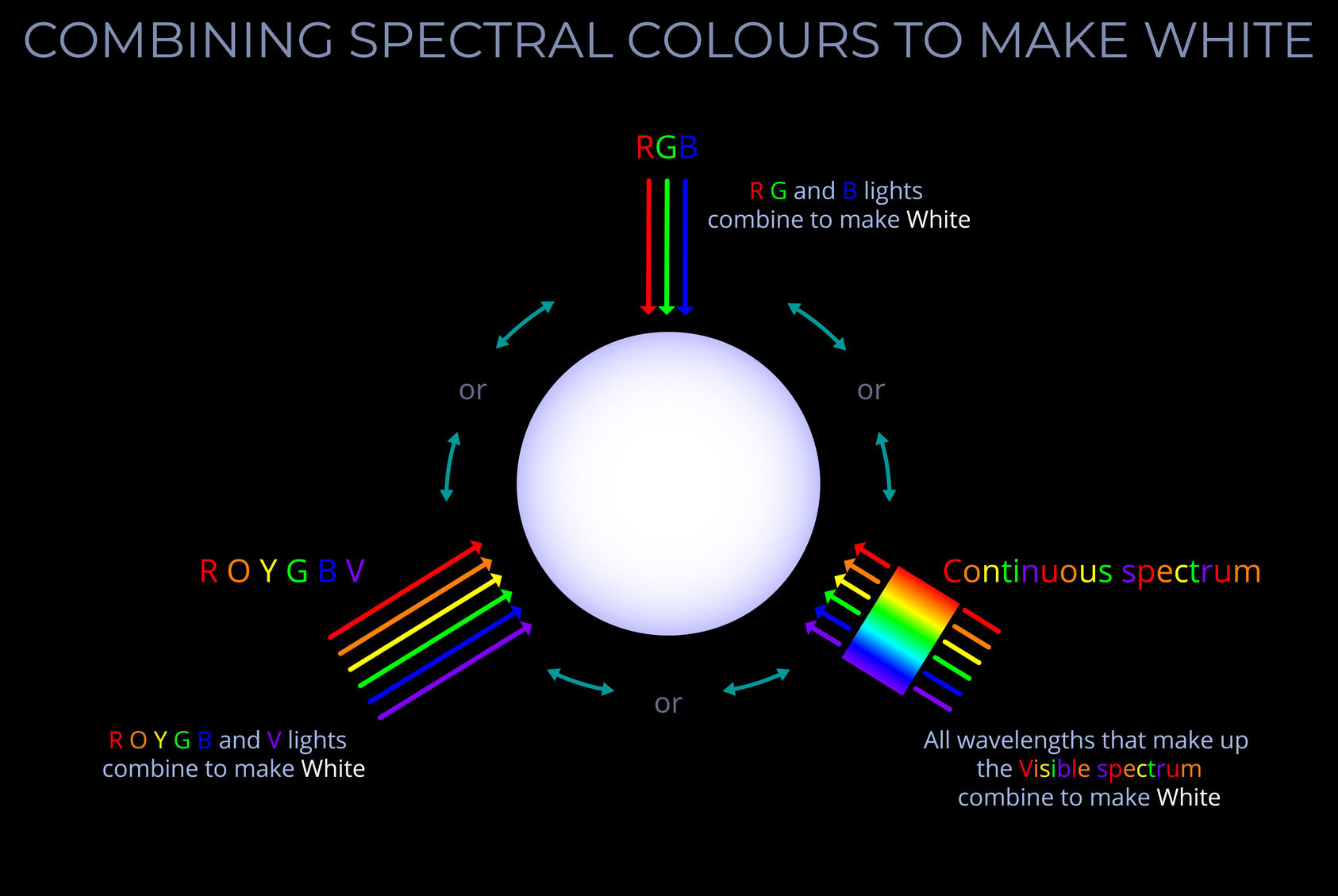
- Whilst the term light can be used to refer to the whole electromagnetic spectrum, visible light refers to the small range of wavelengths our eyes are tuned to.
- The term light can be used in three different ways:
- Light can be used to mean the whole of the electromagnetic spectrum from radio waves, through visible light to gamma rays. When this meaning is intended, the terms radiant energy or photon energy are placed in brackets after the term light in this resource.
- Light can be used to mean the range of wavelengths and frequencies that can be detected by the human eye. A better term is visible light which refers to the wavelengths that correspond with the colours between red and violet, the visible spectrum.
- Light can also be used to mean the range of wavelengths and frequencies between infra-red and ultra-violet. This usage is sometimes useful because the outer limits of the visible spectrum can differ under different lighting conditions and for different individuals.
Light Emission refers to the process by which light (electromagnetic radiation) is produced and emitted by a source. This can occur through various mechanisms, depending on the nature of the source and the conditions involved. These processes involve the transformation of energy into light.
Luminescence
Light is produced when excited electrons within a material drop back to a lower energy state, releasing energy in the form of light. This category includes:
Thermal Radiation
Nuclear Reactions
- Light is produced as a byproduct of nuclear processes like fission (splitting atomic nuclei) and fusion (combining atomic nuclei).
Blackbody Radiation
A light source is a natural or man-made object that emits one or more wavelengths of light.
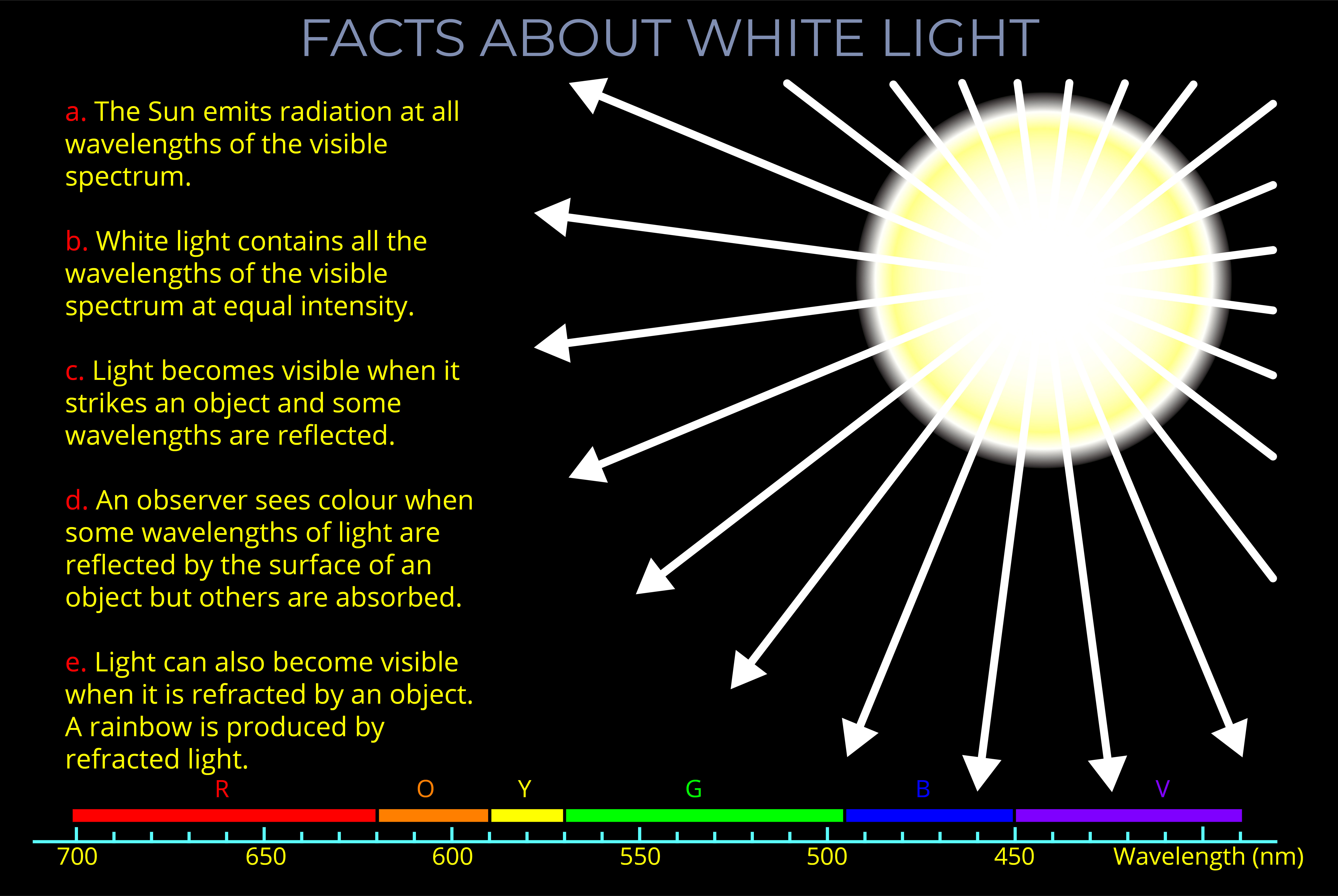
- The Sun is the most important light source in our lives and emits every wavelength of light in the visible spectrum.
- Celestial sources of light include other stars, comets and meteors.
- Other natural sources of light include lightning, volcanoes and forest fires.
- There are also bio-luminescent light sources including some species of fish and insects as well as types of bacteria and algae.
- Man-made light sources of the most simple type include natural tars and resins, wax candles, lamps that burn oil, fats or paraffin and gas lamps.
- Modern man-made light sources include tungsten light sources. These are a type of incandescent source which means they radiate light when electricity is used to heat a filament inside a glass bulb.
- Halogen bulbs are more efficient and long-lasting versions of incandescent tungsten lamps and produce a very uniform bright light throughout the bulb’s lifetime.
- Fluorescent lights are non-incandescent sources of light. They generally work by passing electricity through a glass tube of gas such as mercury, neon, argon or xenon instead of a filament. These lamps are very efficient at emitting visible light, produce less waste heat, and typically last much longer than incandescent lamps.
- An LED (Light Emitting Diode) is an electroluminescent light source. It produces light by passing an electrical charge across the junction of a semiconductor.
- Made-made lights can emit a single wavelength, bands of wavelengths or combinations of wavelengths.
- An LED light typically emits a single colour of light which is composed of a very narrow range of wavelengths.
Light sources
| Emission mechanism | Description | Examples |
| | | |
| LIGHT-EMITTING PROCESS | | | |
| Luminescence | Light emission due to the excitation of electrons in a material. | Electrons within a material gain energy and then release light as they return to a lower energy state. | Bioelectroluminescence
Electroluminescence
Photoluminescence
- Fluorescence
- Phosphorescence
Sonoluminescence
Thermoluminescence
|
| Blackbody radiation (Type of thermal radiation) | Electromagnetic radiation (including visible light) emitted by any object with a temperature above absolute zero. | Electromagnetic radiation (including visible light) emitted by any object with a temperature above absolute zero. | All objects above temperature of absolute zero. |
| Chemiluminescence | Light from natural and artificial chemical reactions. | Light from natural and artificial chemical reactions. | Bioluminescence
Chemiluminescent reactions:
- Luminol reactions
- Ruthenium chemiluminescence |
| Nuclear reaction | Light emission as a byproduct of nuclear reactions (fusion or fission). | Light emitted as a byproduct of nuclear reactions. | Nuclear reactors
Stars undergoing fusion |
| Thermal radiation | Light emission due to the thermal excitation of atoms and molecules at high temperatures. | Light emission due to the thermal excitation of atoms and molecules. | Sun
Stars
Incandescent light bulbs |
| Triboluminescence | Light emission due to mechanical stress applied to a material. | Light emission due to the mechanical stress applied to a material, causing the movement of electric charges and subsequent light emission. | Sugar crystals cracking
Adhesive tape peeling
Quartz crystals fracturing. |
| | | |
| Natural light source | | | |
Fireflies
Deep-sea creatures
Glowing mushrooms | Bioluminescence | Light emission from biological organisms. | Involves the luciferase enzyme. |
Sun
Stars | Nuclear Fusion | Light emission as a byproduct of nuclear fusion reactions in stars. | Electromagnetic spectrum (visible light, infrared, ultraviolet). |
Fire
Candles | Thermal radiation | Light emission due to the thermal excitation of atoms and molecules during the combustion of a fuel source. | Burning of a fuel source, releasing heat and light. |
| Artificial light source | | | |
Fluorescent lights Highlighters
Safety vests | Chemiluminescence | Light emission from chemical reactions. | Fluorescence (absorption and re-emission of light). |
Glow sticks
Emergency signs | Chemiluminescence | Light emission due to phosphorescence - a type of chemiluminescence. | A type of chemiluminescence where light emission is delayed after the initial excitation. |
Glow sticks
Light sticks | Chemiluminescence | Chemiluminescence | Light emission from a chemical reaction that does not involve combustion. |
Tungsten light bulbs
Toasters | Thermal radiation | Heated filament radiates light and heat. | Light emission from a hot filament. |
Fluorescent lamps
LED lights | Electroluminescence | Excitation of atoms by electric current. | Light emission when electric current excites atoms in a material. |
| Neon signs | Electrical Discharge | Discharge of electricity through gas. | Light emission when electricity flows through a gas. |
Sugar crystals cracking
Pressure-sensitive adhesives | Triboluminescence | Light emission from friction or pressure. | Light emission due to mechanical forces. |
Fluorescent paint Highlighters
Safety vests | Photoluminescence | Absorption and subsequent re-emission of light at a lower energy. | Absorption and re-emission of light. |
Light Sources: Mechanism, examples, and everyday applications
Footnote: Cerenkov radiation and Synchrotron radiation are not included in the table because they are not conventionally classified as light sources.
The best light source for a rainbow is a strong point source such as sunlight. Sunlight is ideal because it is so intense and contains all the wavelengths that make up the visible spectrum.
- A human observer with binocular vision (two eyes) has a 1200 field of view from side to side. In clear conditions, the Sun can be considered to be a point-source filling just 0.50 of their horizontal field of view.
- A wide range of visible wavelengths of light is needed to produce all the rainbow colours. The Sun produces a continuous range of wavelengths across the entire visible spectrum.
- When atmospheric conditions like cloud or fog cause too much diffusion of sunlight before it strikes a curtain of rain, no bow is formed.
- Artificial light sources such as LED’s, incandescent light bulbs, fluorescent lights and halogen lamps all make poor light sources because they emit too narrow a range of wavelengths and don’t emit sufficient energy.
Light stimuli trigger physiological responses in living organisms, such as vision, photosynthesis, and circadian rhythms.
- Different organisms respond differently to light stimuli, depending on the presence or absence of specialized light-sensitive cells or photoreceptors.
- Light that enters the human eye and stimulates the visual system is called a visual stimulus.
- The term colour stimulus is used because the light stimulus produces the perception of colour for an observer.
- Every light stimulus can be described in terms of the composition and intensity of wavelengths of light that enter the eye.
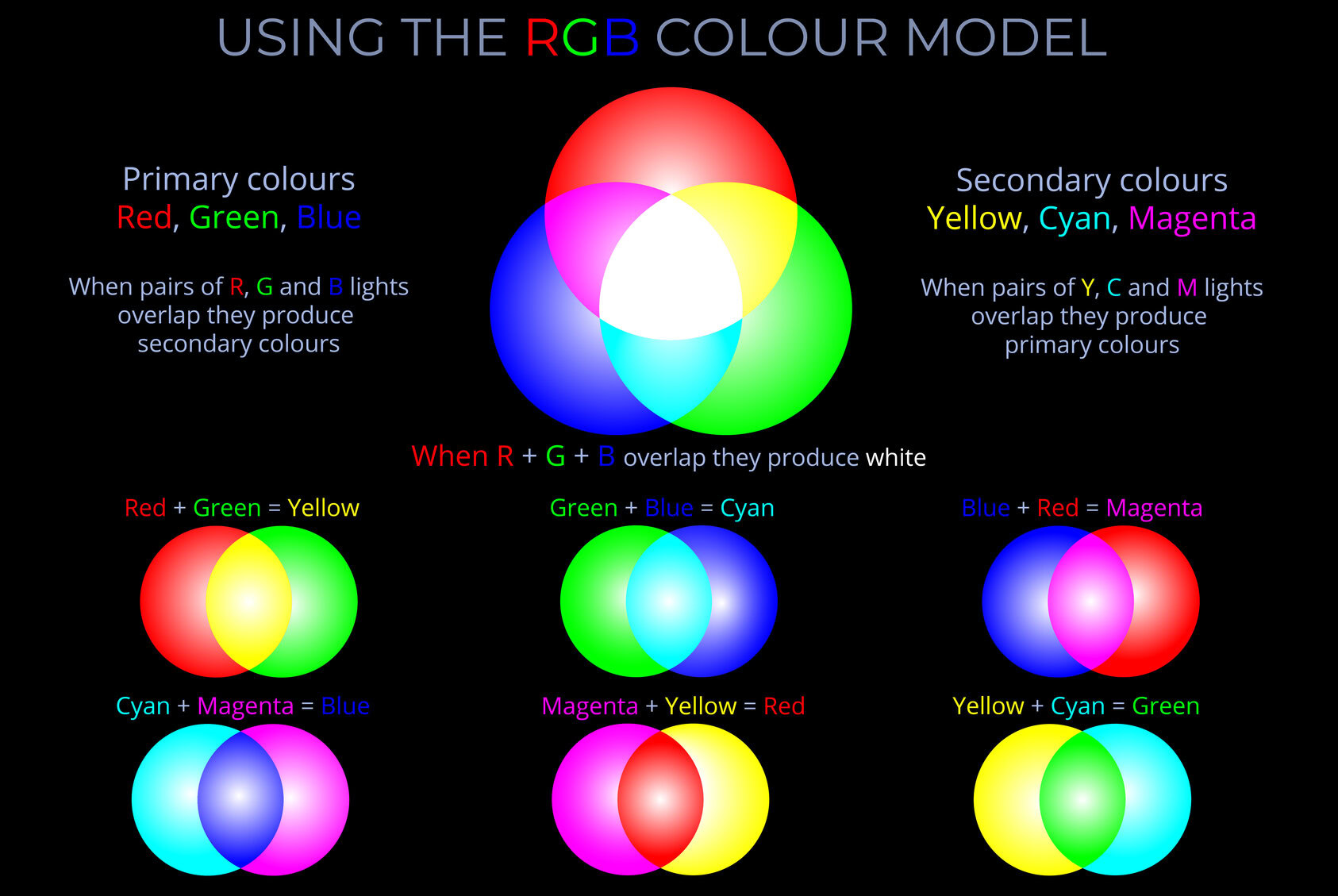
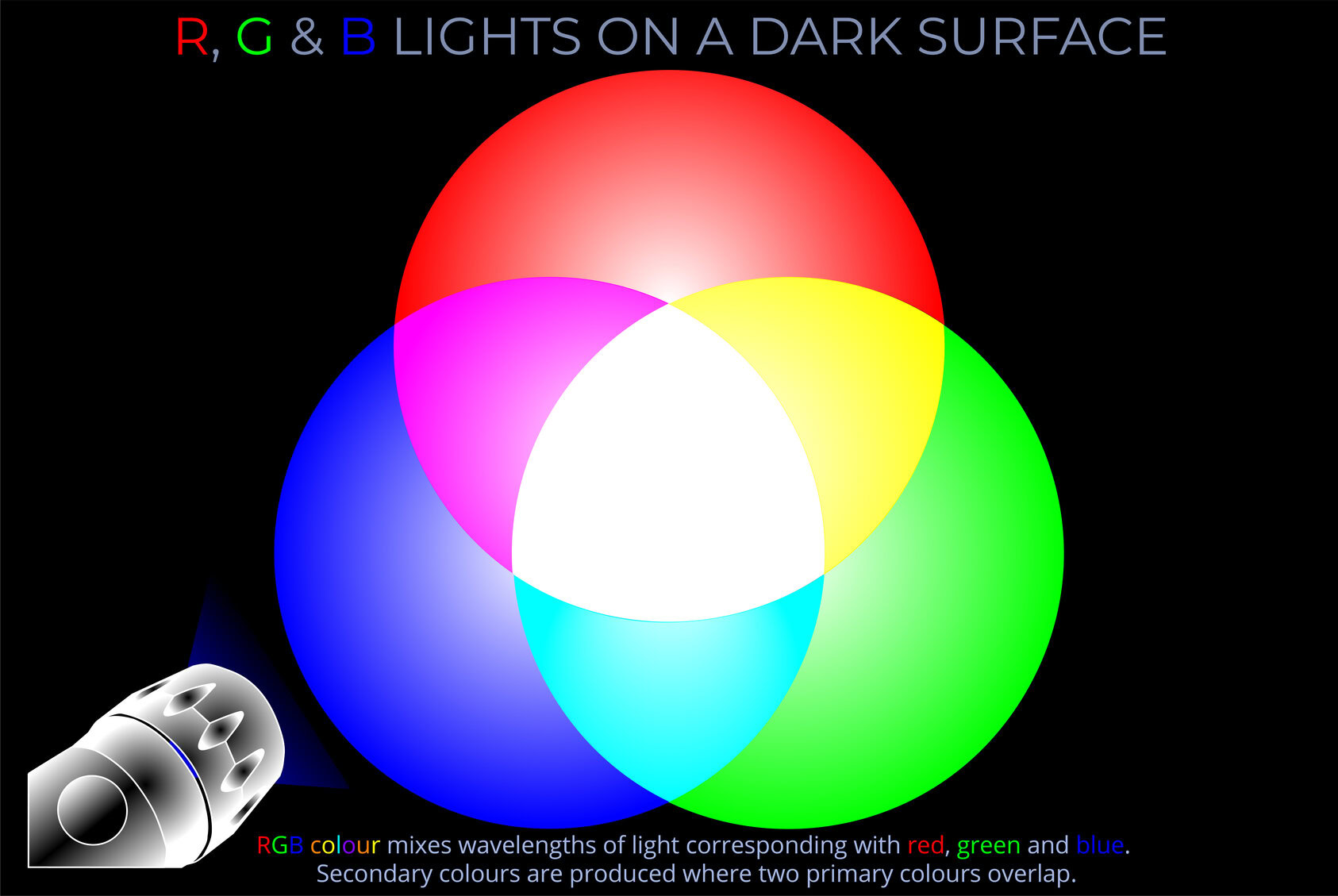
- A light stimulus may consist of a combination of red, orange, yellow, green, blue, and violet wavelengths of light. The colour perceived by an observer is influenced not only by the mixture of wavelengths but also by the intensity of light at each wavelength.
- In many cases, the intensity of light varies across a range of wavelengths, and this variation can be described by the spectral power distribution of the stimulus.