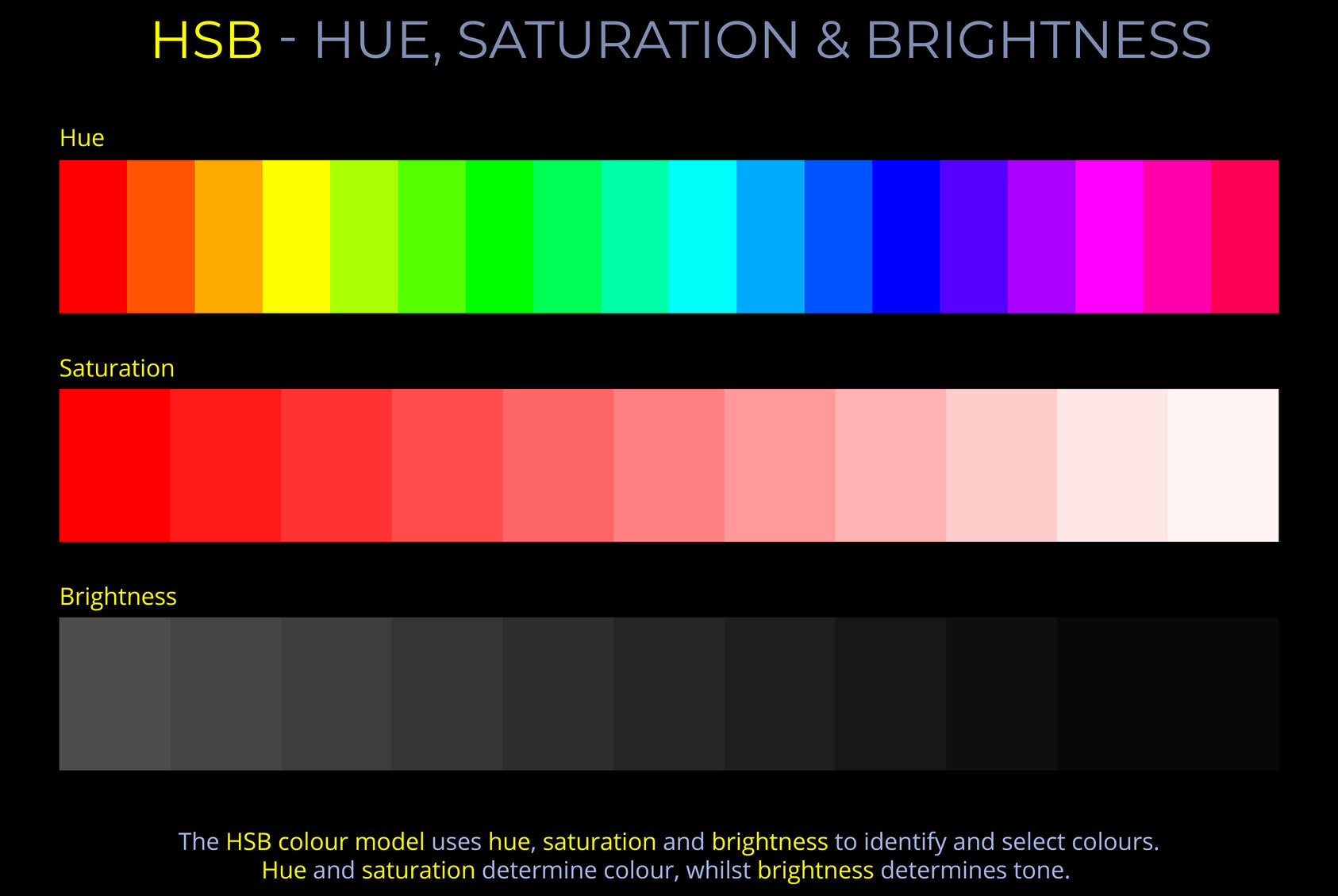
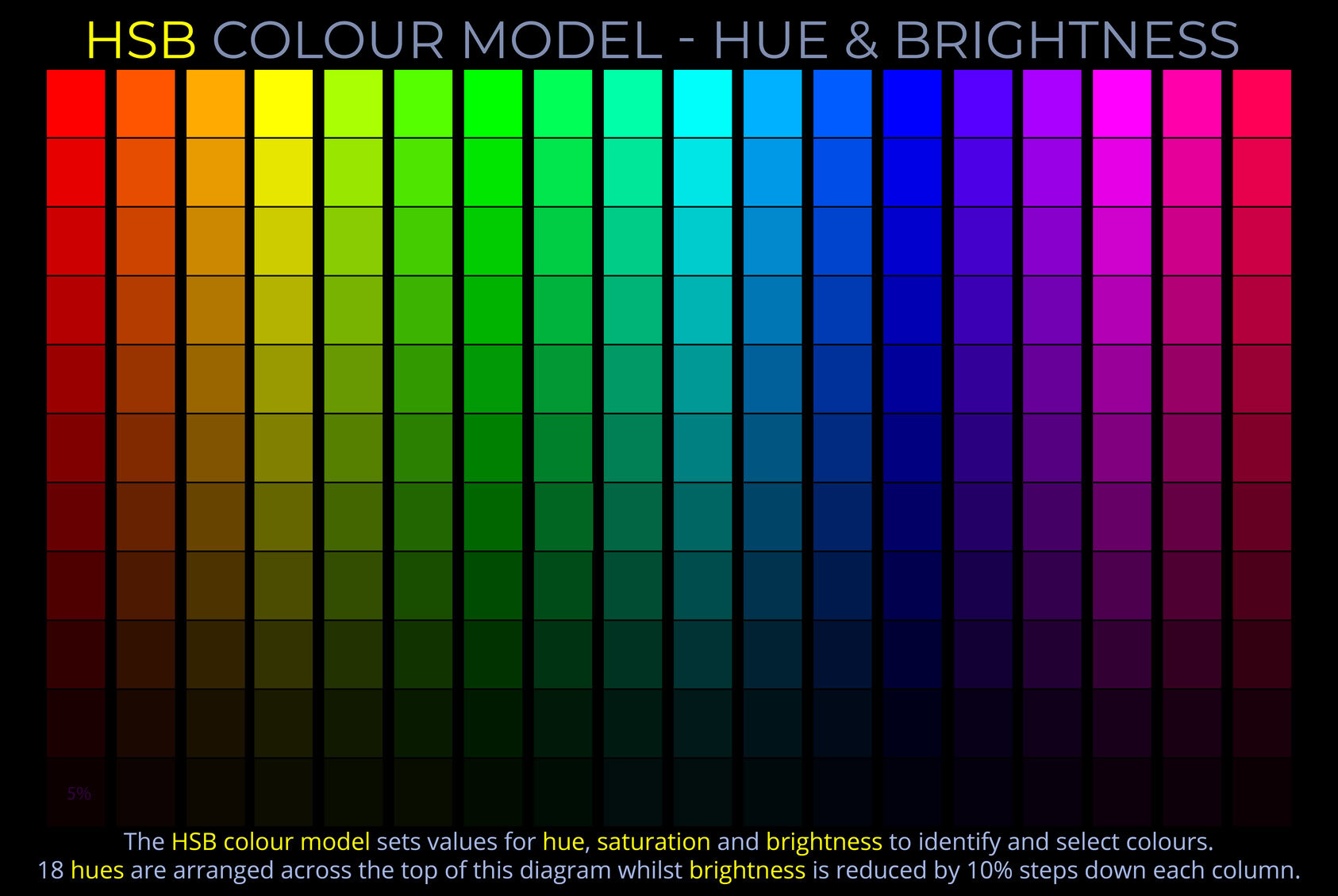
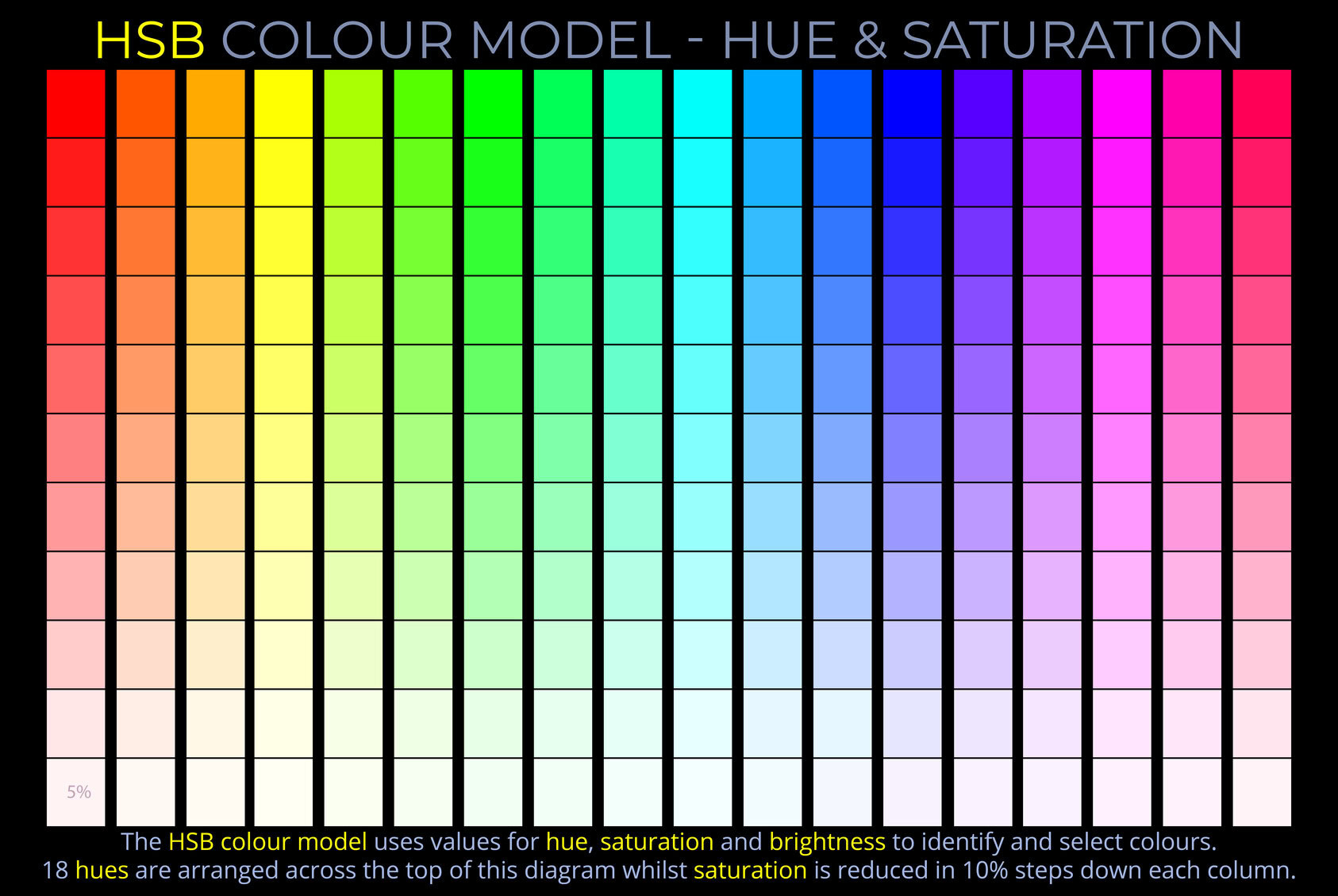
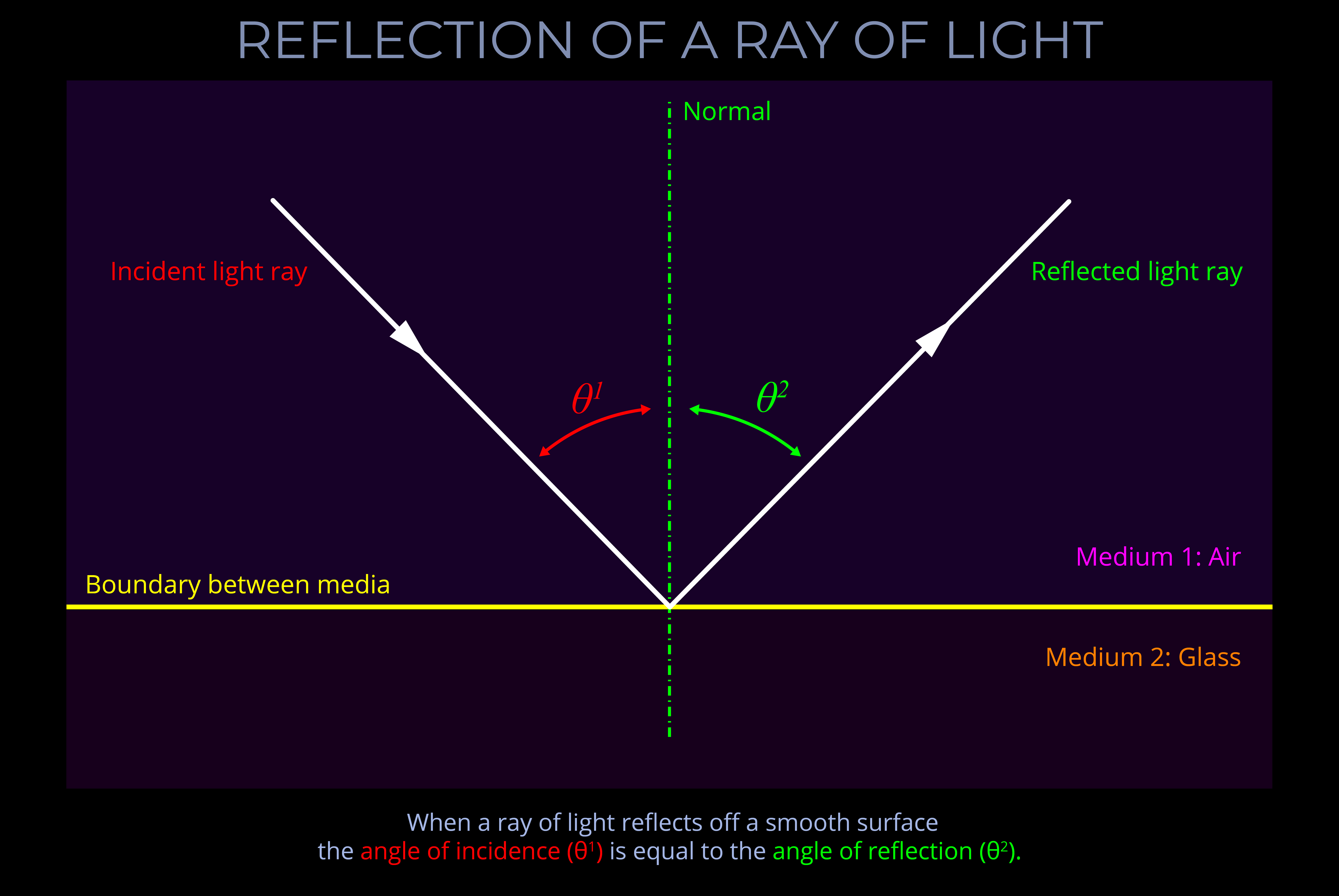
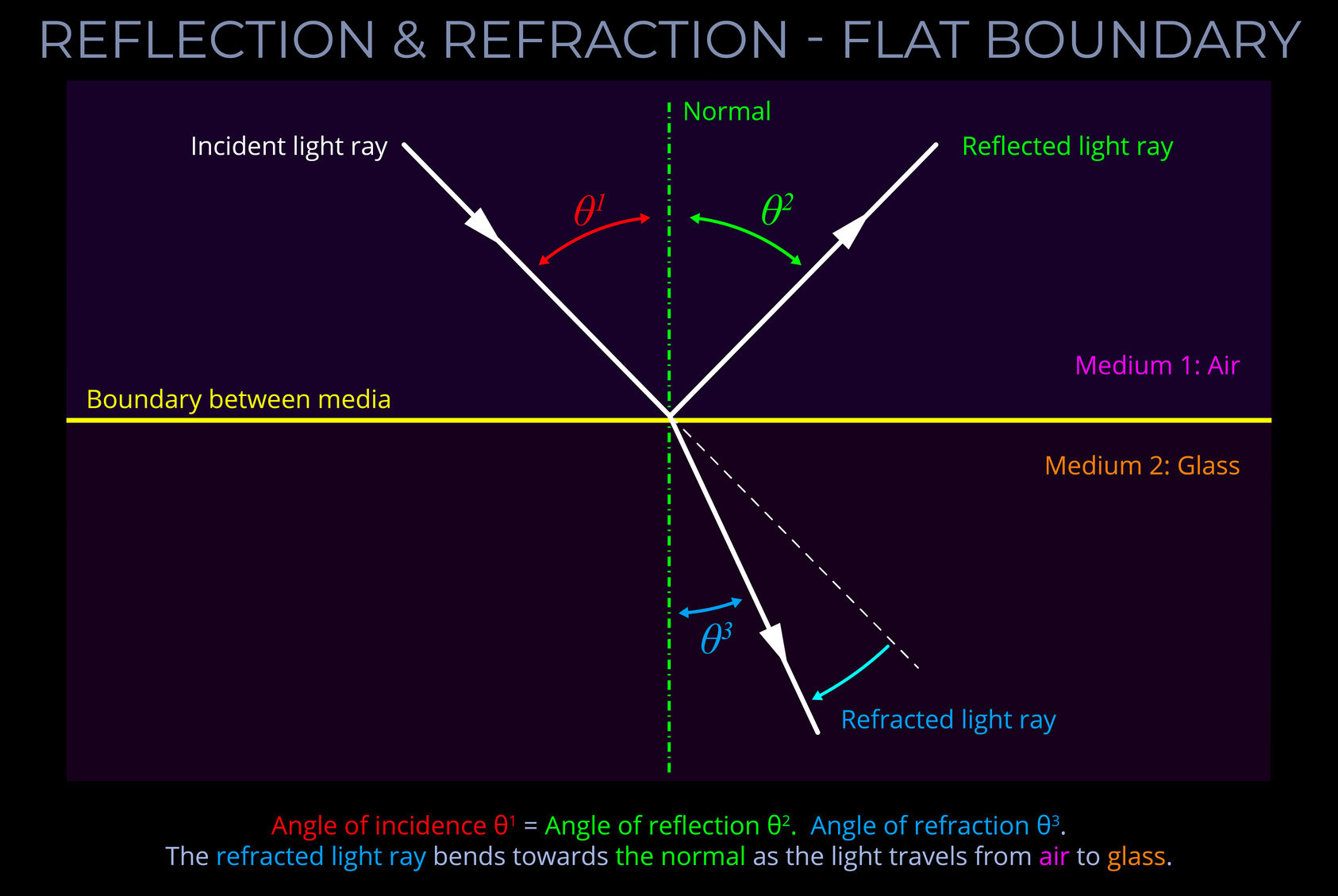
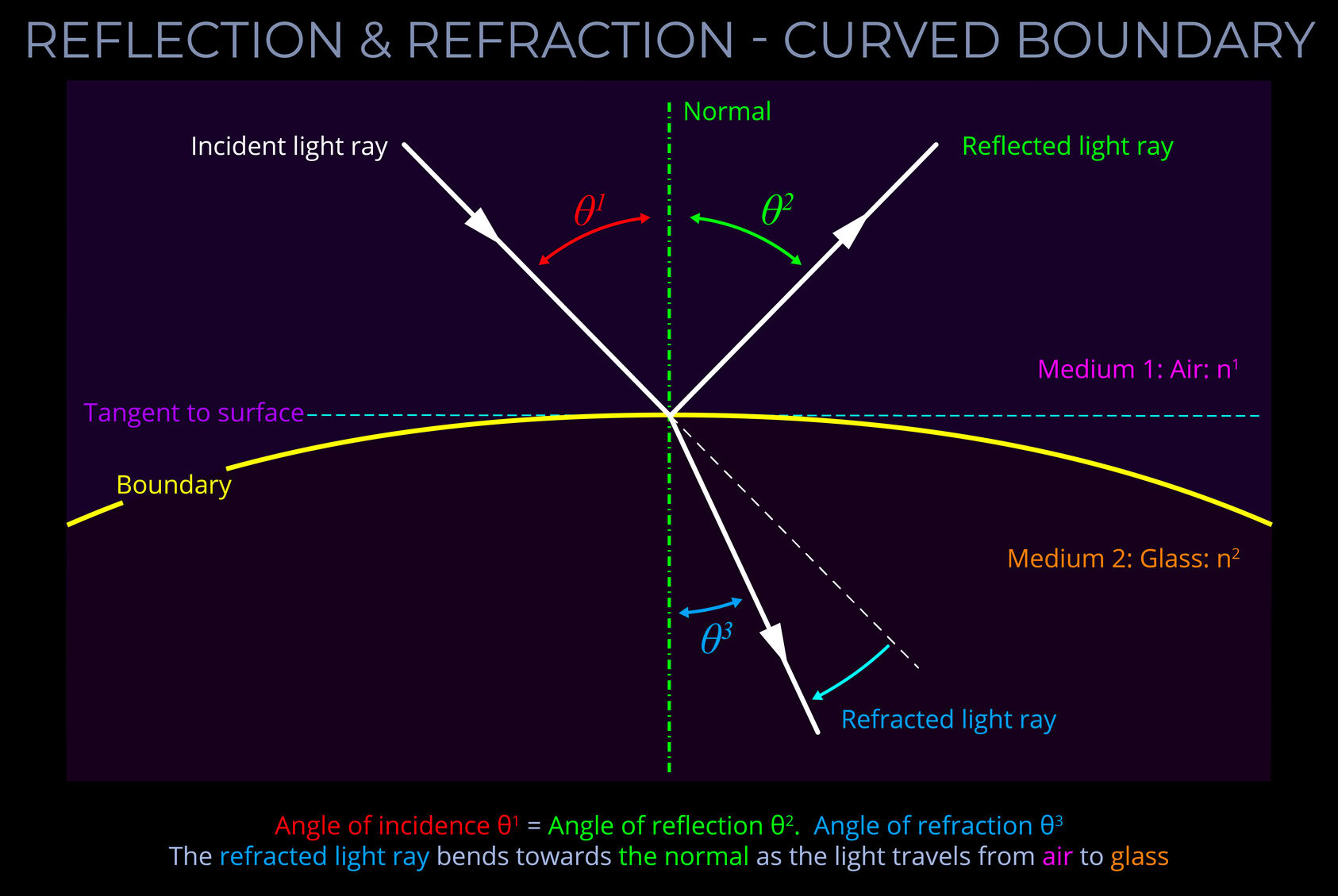
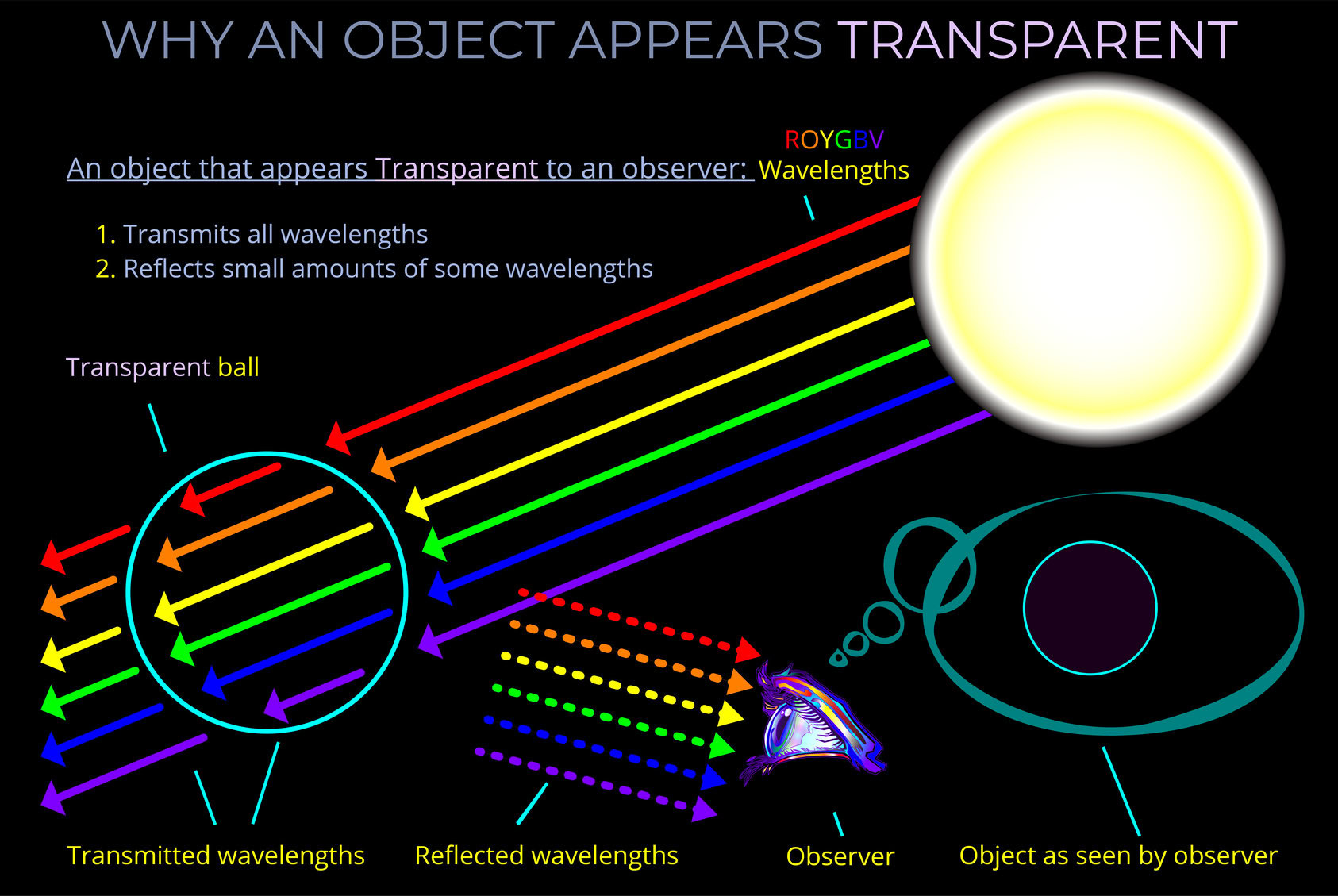
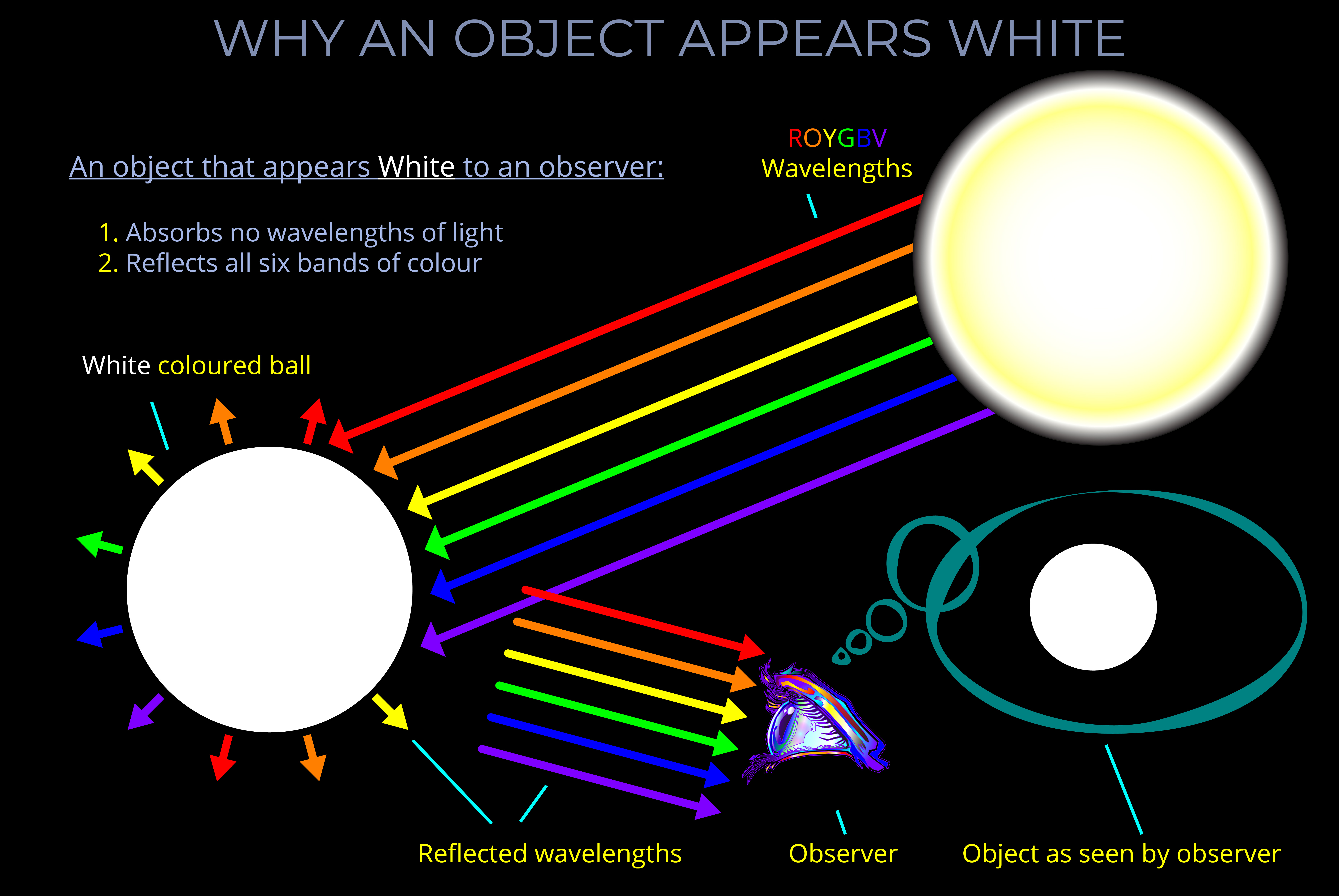
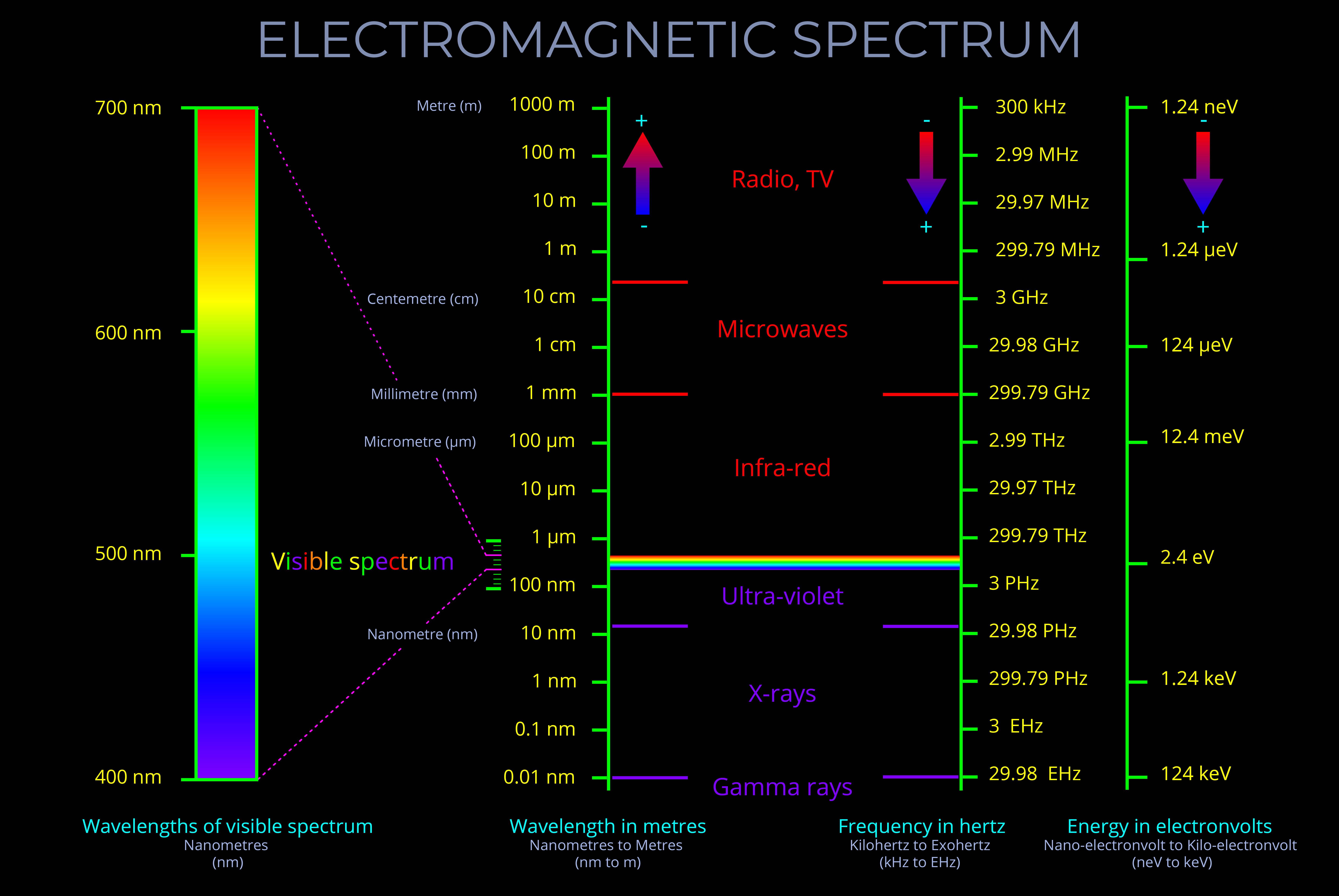
The LMS colour space is a practical implementation of trichromatic colour theory that enables the full range of human observable colours to be specified by measuring the responsiveness of the L, M and S cones to each wavelength of light within the visible spectrum.
- The LMS colour space was one of the first systematic demonstrations of trichromatic colour theory.
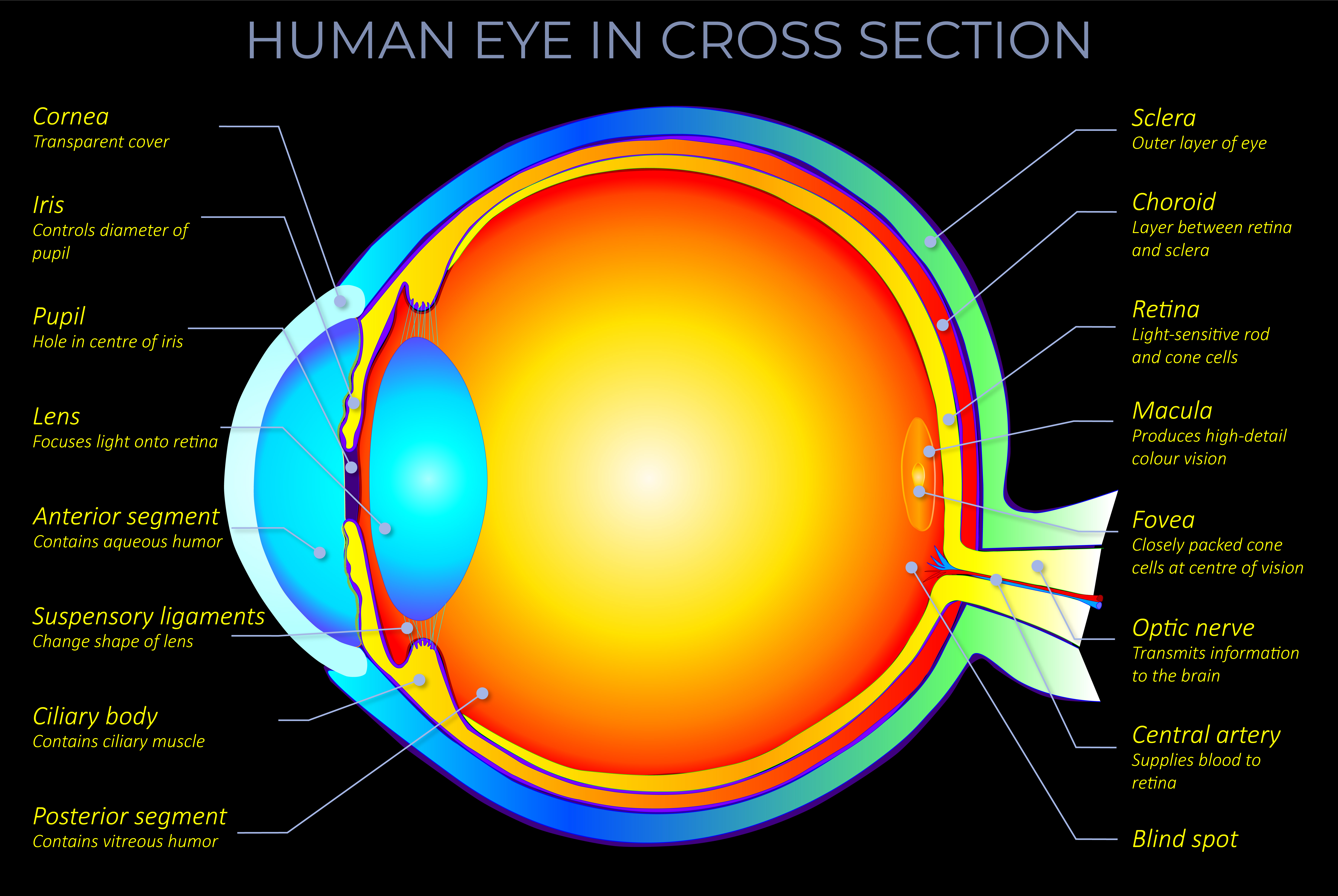
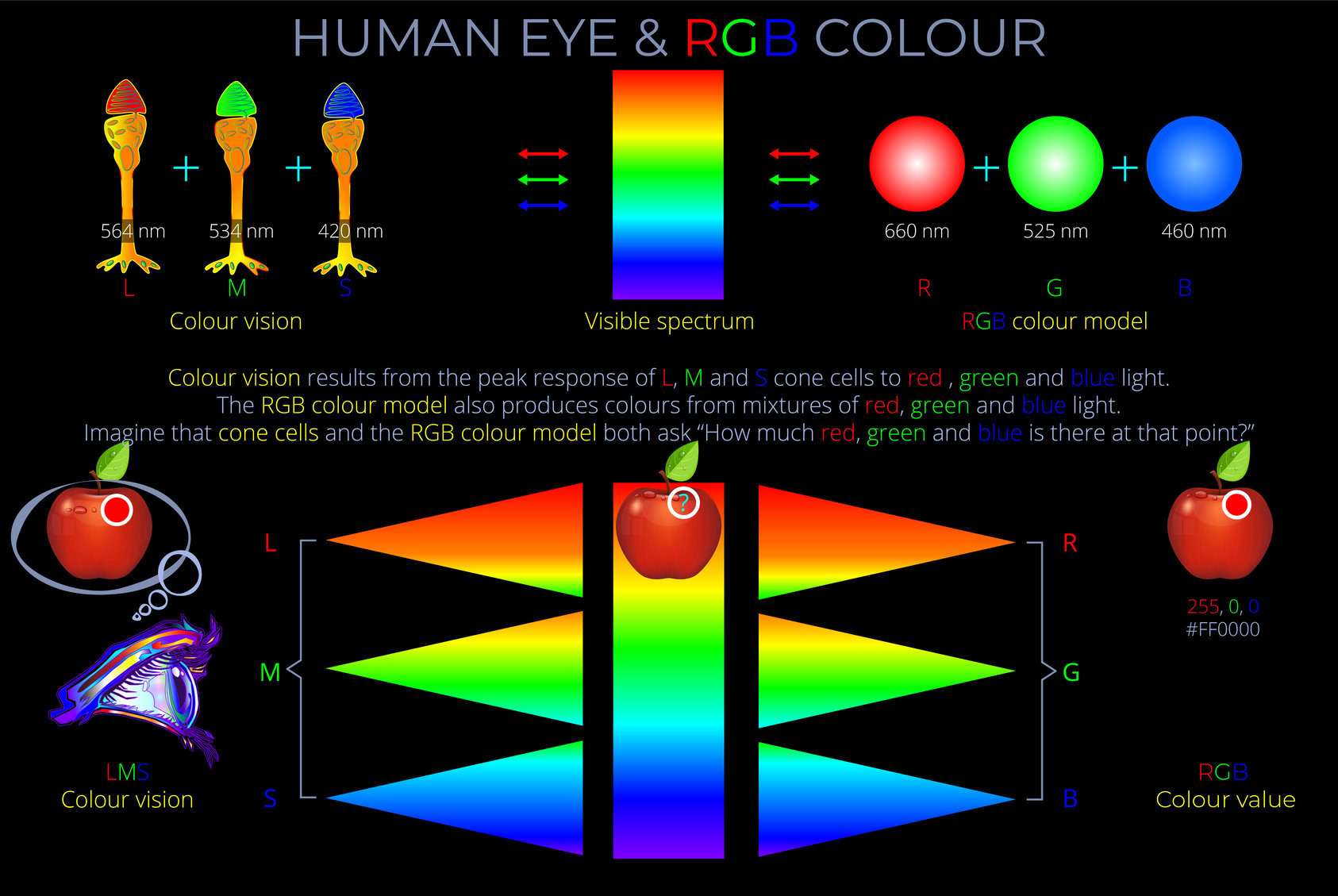
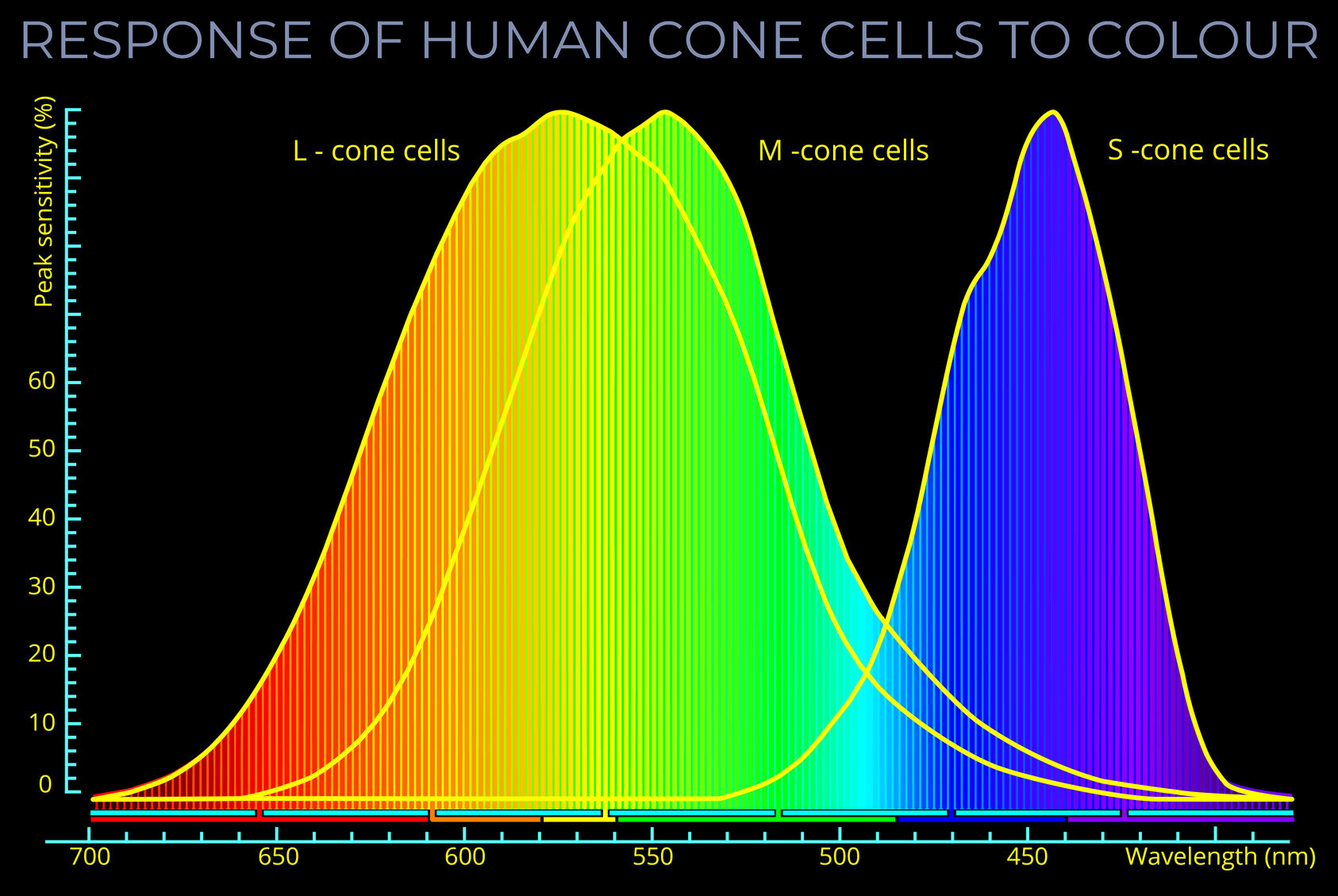
- LMS describes how the three types of cone photoreceptors (L, M and S cone types) in a human eye respond given any particular light stimuli.
- The method used in the development of the LMS colour space produced a generalized representation of human colour perception.
- The underlying principle was that any colour can be described in physiological terms by measuring the response of the L, M and S cone cells in the human eye’s retina to different wavelengths of light.
- The initial source of data for the LMS colour space was taken from experiments that compared the spectral sensitivity of subjects with normal sensitivity with other subjects experiencing forms of colour blindness.
- A more recent technique used to collect data for LMS belongs to the field of visual psychophysics and is known as heterochromatic flicker photometry. It provides extensive and accurate spectral sensitivity data obtained from cellular material removed from the eye.
- The LMS colour space describes human observable colours using three parameters, known as tristimulus colour values, each component of which corresponds with the response of the L, M and S cone types.
Tristimulus colour values
- Tristimulus colour values have three components corresponding with the response of the L, M and S cone types. Each response is measured against a scale with values between 0 and 1.
- LMS tristimulus colour values for a monochromatic red, green and blue stimulus might appear as follows:
- Red: wavelength = 635 nanometres: L = 0.3278612, M = 0.0444877, S = 0.0
- Green: wavelength = 520 nanometres: L = 0.6285647, M = 0.8166012, S = 0.02920317
- Blue: wavelength= 450 nanometres: L = 0.04986433, M = 0.08705225, S = 0.9553885
- Data from: https://cie.co.at/datatable/cie-2006-lms-cone-fundamentals-2-field-size-terms-energy
- Tristimulus colour values can be thought of as colour-matching functions. If you know a tristimulus colour value then you can predict the corresponding colour experience.
Limitations of the LMS colour space
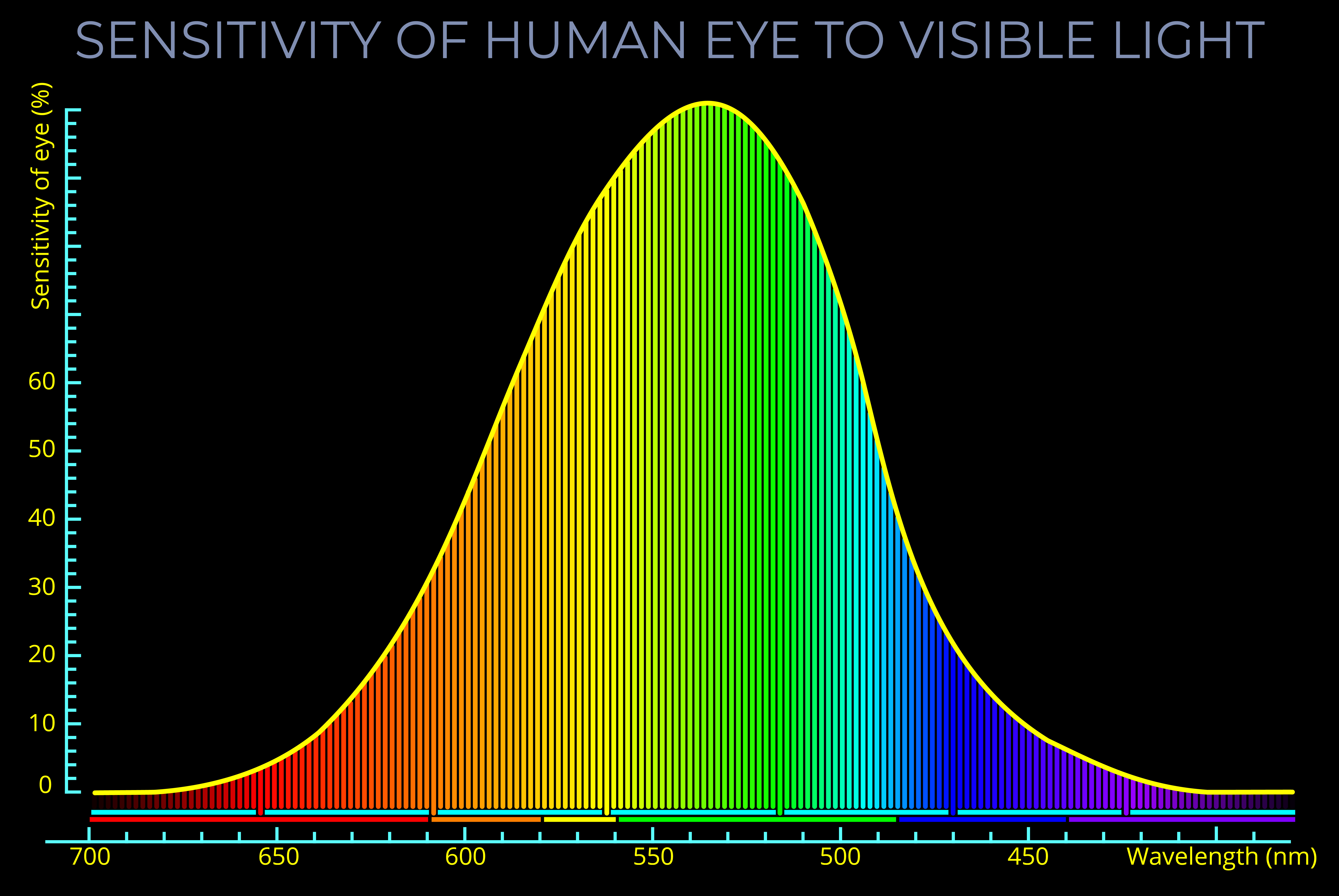
- The LMS colour space provides an accurate physiological description of human colour perception but has limitations related to the fact that some colours in the visible spectrum appear brighter than others.
- Whilst the achievements of the research that produced the LMS colour space underpin much of the subsequent developments within the field, LMS has been superseded by the CIE (1931) XYZ colour space.
- The CIE (1931) XYZ colour space addresses the limitations of the LMS colour space by sacrificing physiologically accurate measurements of colour perceptions in favour of a solution better suited to everyday colour management..
- The XYZ tristimulus colour parameters replace the LMS tristimulus colour parameters
- XYZ is a more convenient representation and the CIE XYZ colour plot defines all the possible colours a human observer can see. For a given luminance Y, the XZ value specifies all possible chromaticity values one can see.
- There is a simple linear transformation, via a 3 x 3 matrix, between LMS and XYZ.
References
- https://en.wikipedia.org/wiki/Trichromacy
- https://en.wikipedia.org/wiki/LMS_color_space
- https://en.wikipedia.org/wiki/CIE_1931_color_space
- https://www.sciencedirect.com/topics/engineering/color-matching-function
- https://www.strollswithmydog.com/cone-fundamental-lms-color-space/
- http://cvrl.ucl.ac.uk/people/Stockman/pubs/2006%20Physiological%20CMFs%20SS.pdf
- https://www.cns.nyu.edu/~david/courses/perception/lecturenotes/color/color.html