A nanometre (nm) is a unit of length in the metric system, equal to one billionth of a metre (1 nm = 1 × 10⁻⁹ metres). It is commonly used to measure extremely small distances, particularly at the atomic and molecular scale.
- In the context of light and electromagnetic radiation, a nanometre is often used to describe wavelengths of visible light.
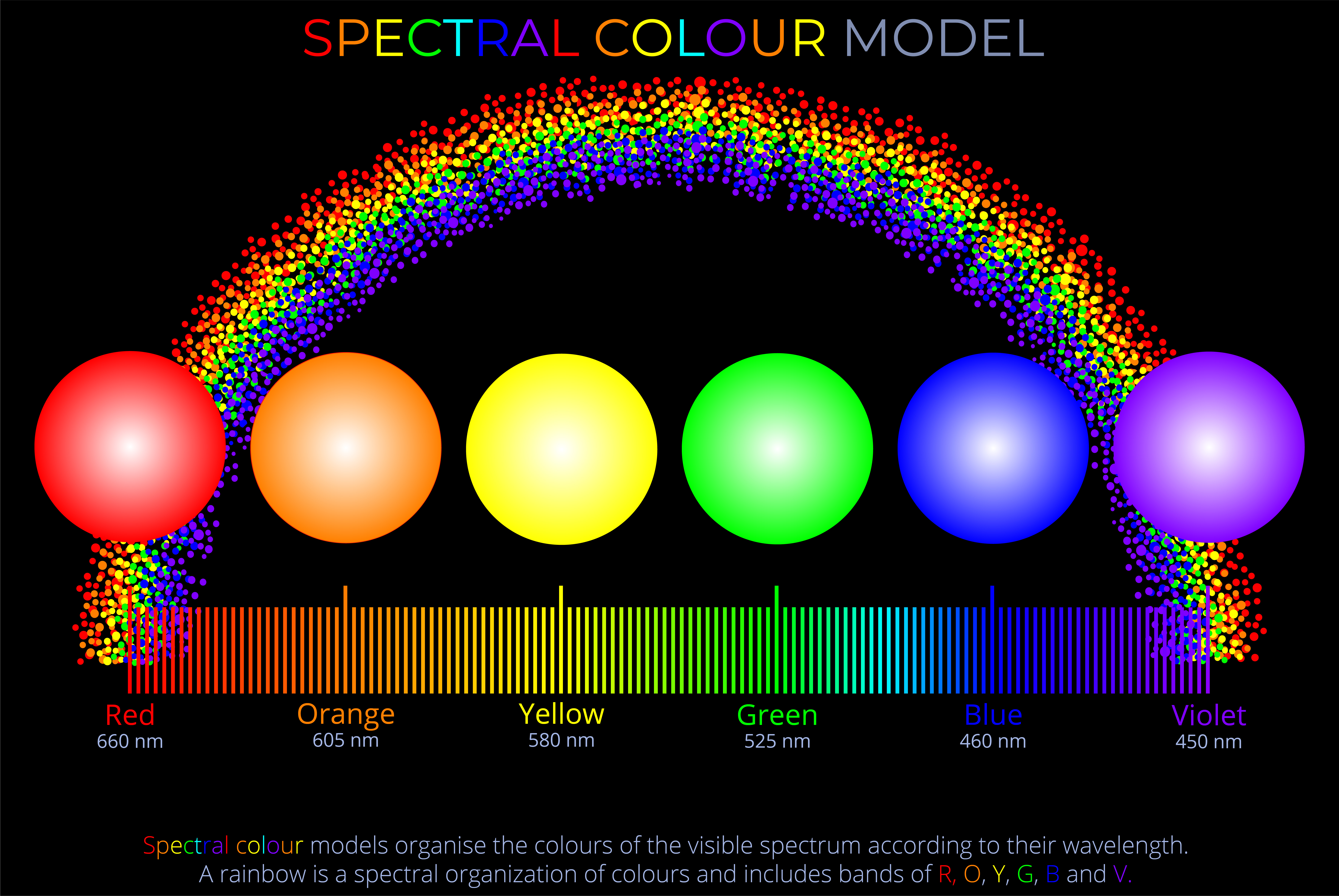
The wavelength of visible light ranges from about 700 nm (red) to 400 nm (violet).
- Nanometres are also used to measure components like the thickness of materials, the size of particles in nanotechnology, and the spacing between atoms in a crystal lattice.
A natural light source refers to any source of light that occurs in nature and is not created by human activity.
- The Sun is the most prominent and important natural light source on Earth, providing sunlight that powers life, such as through photosynthesis in plants.
- Stars emit light naturally due to nuclear reactions in their cores, which generate massive amounts of energy released as light.
- Fire can occur naturally through processes like lightning strikes igniting dry vegetation or volcanic activity.
- Bioluminescence is the natural emission of light by living organisms such as fireflies, some fungi, and deep-sea creatures.
- Auroras (like the Northern and Southern Lights) are natural light displays in the Earth’s atmosphere, caused by the interaction of solar wind with the Earth’s magnetic field.
- Lightning is another natural light source, produced during electrical storms when electrical charges in clouds discharge.
- Natural light sources vary in brightness, spectrum, and duration.
Nature, in the broad sense, refers to the physical universe encompassing all living organisms (plants, animals, microorganisms) and non-living entities (such as rocks, water, and atmospheric elements). It includes the natural processes and forces that govern the physical world, as well as ecosystems and the interactions between living and non-living components.
- Nature, in the broadest sense, refers to the physical universe, encompassing all living and non-living things.
- In a more limited sense, nature can refer specifically to interconnected living organisms, including plants, insects, and animals, while sometimes excluding non-living elements like oceans, continents, and climate.
- However, it’s important to note that non-living phenomena are also an essential part of nature, as they play a vital role in ecosystems and the natural processes that sustain life.
- The concept of nature is complex and multifaceted. For instance, while humans are part of nature, human-made environments such as cities, agriculture, and industries are often viewed as distinct from other natural phenomena.
Neurons are specialized cells that transmit electrical and chemical signals throughout the brain and central nervous system, enabling communication between different parts of the body the central nervous system.
- Neurons are the electrically excitable cells that are the fundamental building blocks of the central nervous system of human beings.
- Neurons interconnect the systems and organs that maintain the body’s essential functions.
- Neurons send and receive signals that allow us to sense the external world, move, think, form memories and much more.
- Neurons are of three principal types: motor neurons, sensory neurons and interneurons.
- Neurons connect together via specialized filaments called synapses.
- In the neocortex (making up about 80% of the human brain), approximately 70-80% of nervous tissue is in the form of neurons whilst the remainder is composed of interneurons.
Newtonian mechanics is a branch of physics that describes the motion of objects under the influence of forces. It is based on the three laws of motion developed by Isaac Newton in the 17th century.
The three laws of motion are:
- An object at rest will remain at rest, or if in motion, will remain at a constant speed and in a straight line unless acted upon by an external force.
- The acceleration of an object is directly proportional to the net force acting on it, and inversely proportional to its mass.
- For every action, there is an equal and opposite reaction.
- These laws can be used to describe a wide range of phenomena, from the motion of planets to the behaviour of fluids and the propagation of waves. They are also applied in many fields, including engineering, medicine, and astronomy.
- Newtonian mechanics predicts the motion of objects with high accuracy. However, it has limitations; for example, it cannot explain the behaviour of light at atomic and subatomic levels, where light behaves as both a wave and a particle—something Newtonian mechanics cannot describe.
- Despite its limitations, Newtonian mechanics remains a crucial and useful theory. It is applied in many fields and has greatly deepened our understanding of the universe.
Here are examples of Newtonian mechanics in action:
- When you throw a ball, the ball accelerates due to the force of gravity.
- When you ride a bike, you need to pedal to keep moving forward because of the force of friction.
- When you sit in a chair, the chair exerts an upward force on you that balances the downward force of gravity.
- When you jump off a cliff, you accelerate due to gravity until you hit the water.
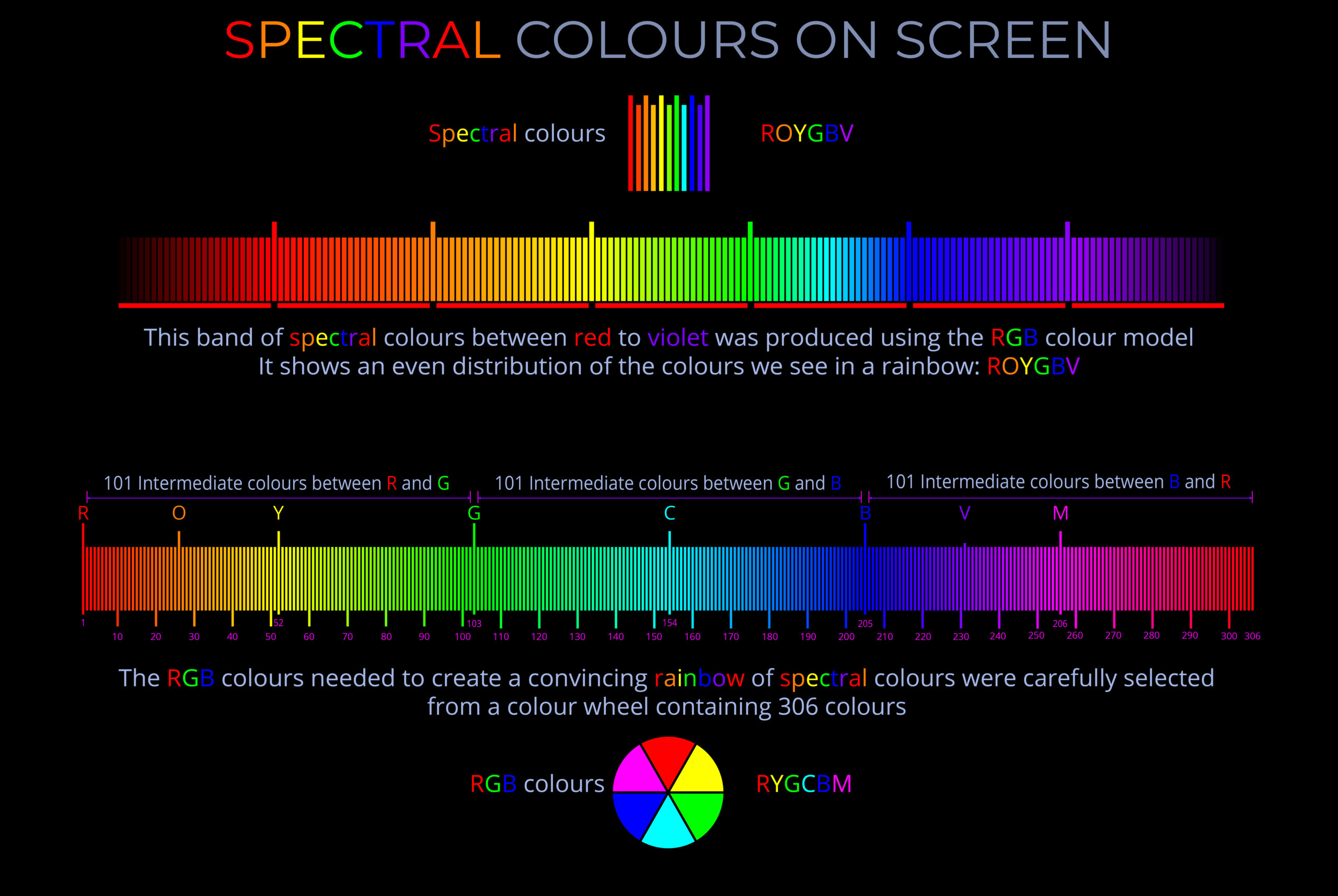
A non-spectral colour is a colour that is not present in the visible spectrum and cannot be produced by a single wavelength or narrow band of wavelengths of light.
- While spectral colours are evoked by a single wavelength of light in the visible spectrum, non-spectral colours are produced by a combination of spectral colours from different parts of the spectrum.
- Colours evoked by a single wavelength of light are often described as being produced by monochromatic light.
- Magenta, pink, cyan and brown are examples of non-spectral colours produced by combining different wavelengths of light:
- Blue and red = magenta
- Red and purple = pink
- Blue and green = cyan
- Red, yellow and blue = brown
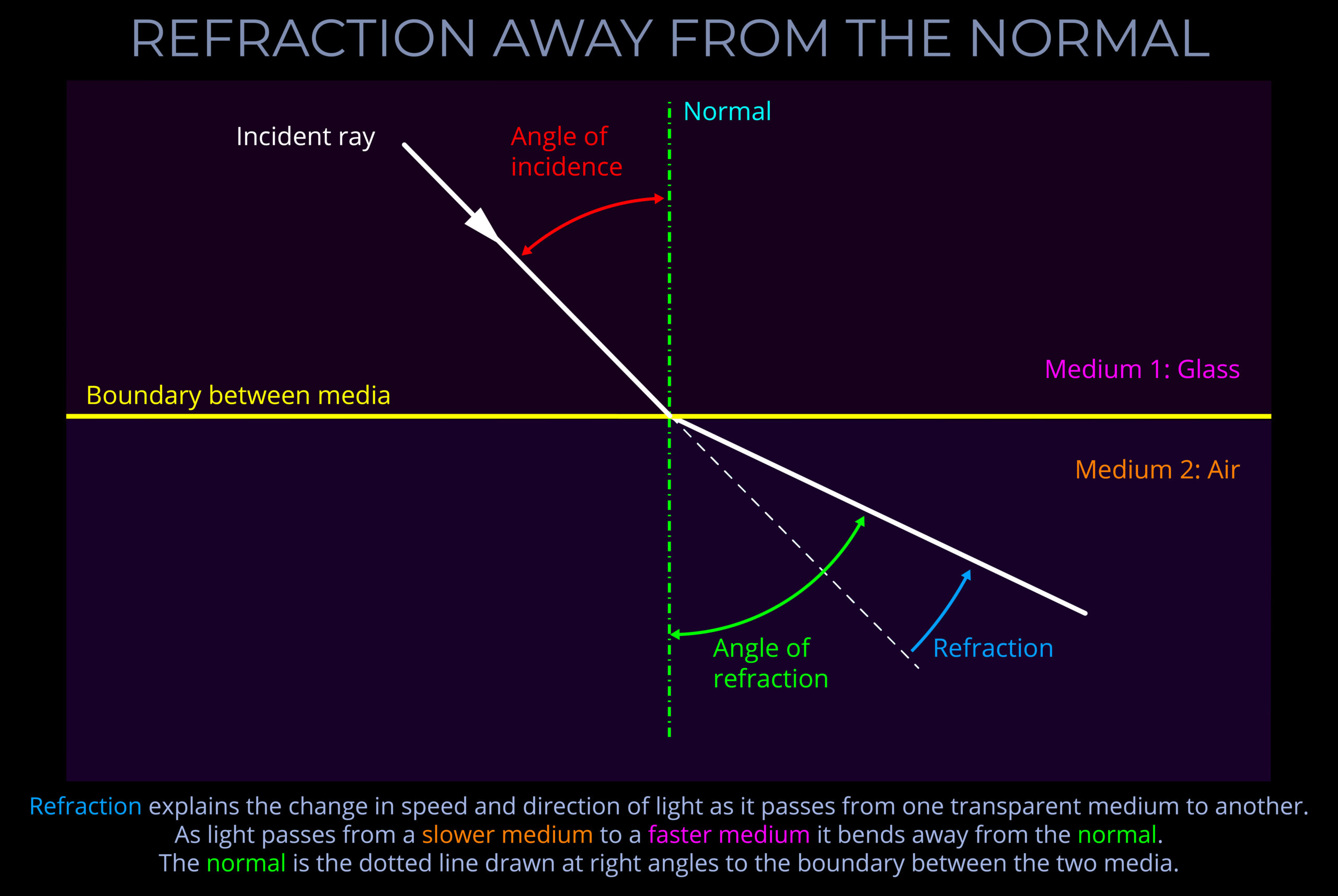
If one line is normal to another, then it is at right angles to it.
In geometry, normal (a or the normal) refers to a line drawn perpendicular to a given line, plane or surface.
- How the normal appears in a geometric drawing depends on the circumstances:
- When light strikes a flat surface or plane, or the boundary between two surfaces, the normal is drawn perpendicular to the surface, forming a right angle (90°) with it.
- Expressed more formally, in optics, the normal is a geometric construct, a line drawn perpendicular to the interface between two media at the point of contact. This conceptually defined reference line is crucial for characterizing various light-matter interactions, such as reflection, refraction, and absorption.
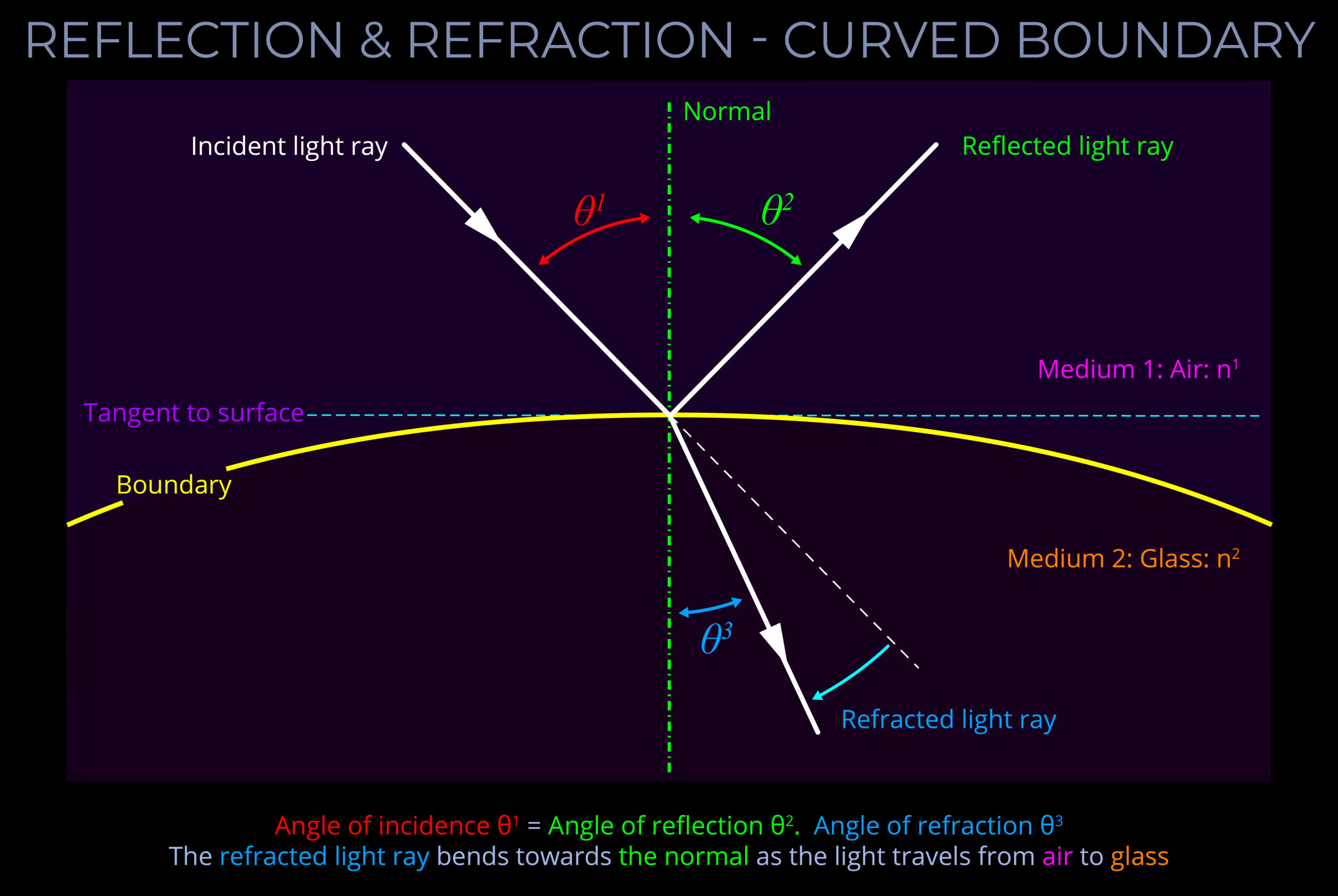
- When dealing with curved surfaces, such as those found on spheres or other three-dimensional objects, determining the normal requires a slightly different approach. Instead of simply drawing a line perpendicular to the surface as with a flat plane, draw the normal straight up from the point where light hits the surface.
- When considering a sphere, the normal line passes through the centre of the sphere. This is because, regardless of where light enters or exits the sphere, the normal represents the direction perpendicular to the surface at that point.
A nuclear reaction involves changes within the nucleus of an atom, resulting in the release of energy and often the emission of particles, as well as electromagnetic radiation. This radiation can span various parts of the electromagnetic spectrum, with gamma rays being a particularly common form.
- Here’s a breakdown of how nuclear reactions can be sources of electromagnetic radiation:
- Nuclear Fission: When the nucleus of a heavy atom splits into smaller nuclei, it releases a significant amount of energy. A significant portion of this energy is emitted as gamma rays, which are high-energy photons within the electromagnetic spectrum. Nuclear power plants and atomic bombs harness fission reactions.
- Nuclear Fusion: When the nuclei of lighter atoms combine to form a heavier nucleus, it also releases energy. In stars like our Sun, nuclear fusion releases large amounts of energy, including a range of electromagnetic radiation from infrared light to ultraviolet light, and even gamma rays.
- Radioactive Decay: Unstable atomic nuclei undergo decay and change their composition to reach a more stable state. During this process, they can release charged particles (like alpha or beta particles), neutrinos, and often gamma rays.
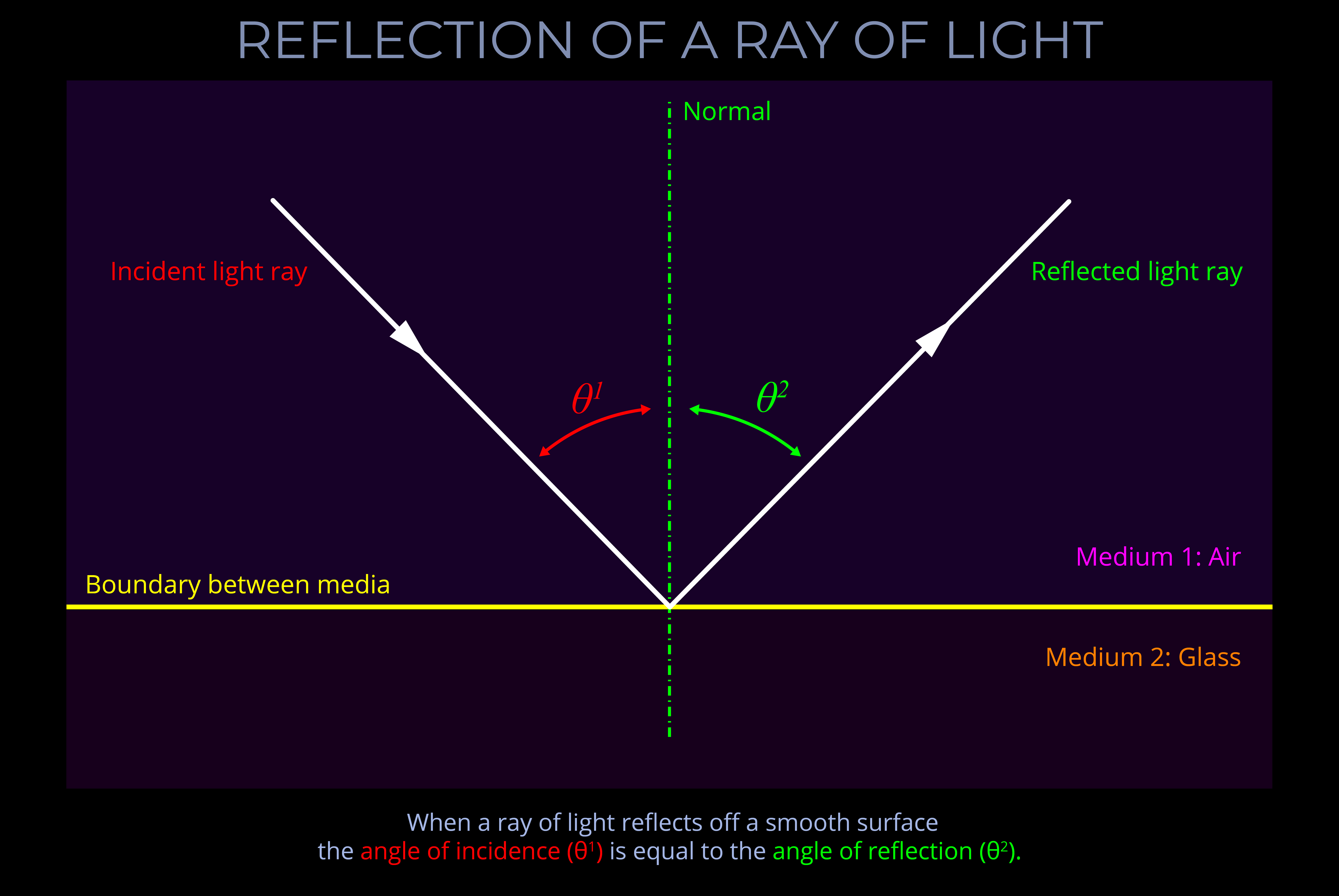
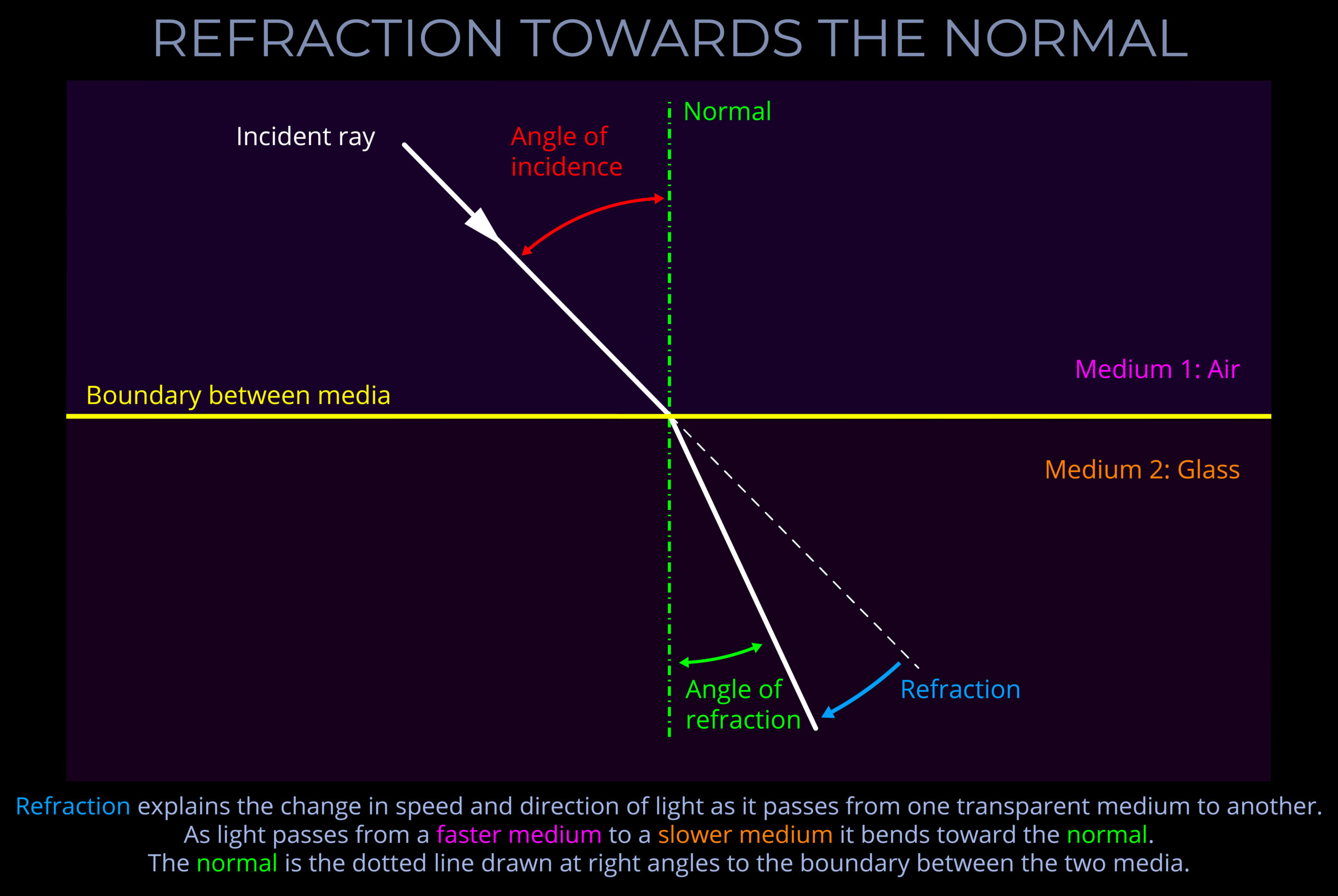
About the normal, angles of incidence, reflection & refraction
-
- The angles of incidence, reflection and refraction are measured between a ray of light and an imaginary line called the normal.
- In, general terms, if one line is normal to another, then it is at right angles to it.
In geometry, normal (a or the normal) refers to a line drawn perpendicular to a given line, plane or surface.
-
- How a normal appears in a geometric drawing depends on the circumstances:
-
- When light strikes a flat surface or plane, or the boundary between two surfaces, the normal is drawn perpendicular to the surface, forming a right angle (90°) with it.
- When light hits a curved surface, the normal line is drawn straight up from the point where the light hits the surface.
- If light travels directly through the centre of a sphere, the normal line also passes through the centre of the sphere.
- When a normal is drawn on a ray-tracing diagram, it provides a reference perpendicular to the surface against which changes in the direction of light can be measured.