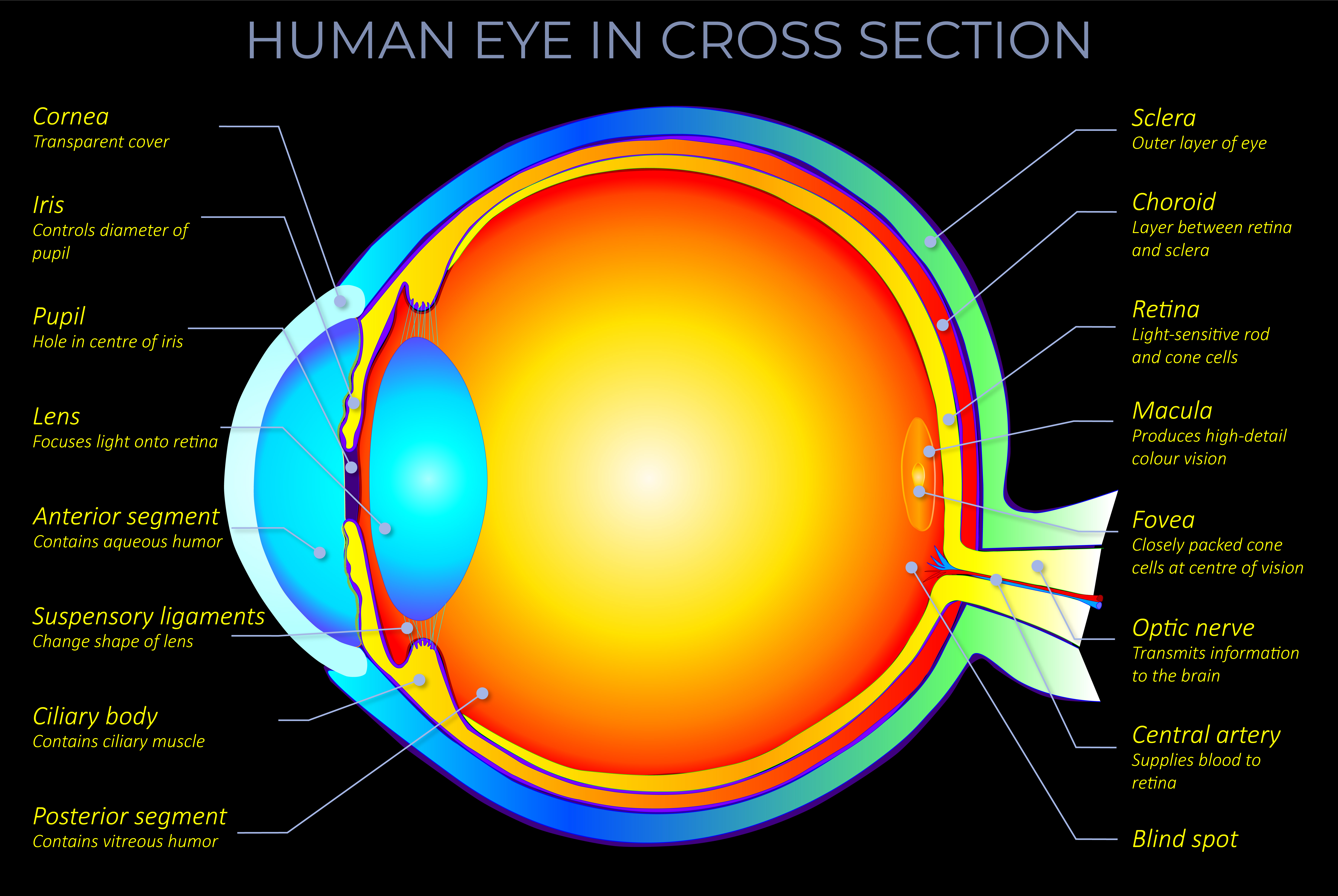
- A single orbital can only contain a maximum of two electrons (Pauli Exclusion Principle) and they can not have the same spin. This means that two electrons in the same orbital must have opposite spins.
- The spin of an electron plays a key role in atomic structure and chemical bonding.
- It is common to illustrate the spin of an electron using clockwise and anti-clockwise arrows. These are an analogy that serves as a way of understanding the two possible spin states.
- A quantum number (ms) represents this property, with +1/2 representing spin up (represented by an arrow pointing clockwise) and -1/2 representing spin down (represented by an arrow pointing anti-clockwise).
- In an atom, electrons are identified and described using four quantum numbers. These numbers provide information about the electron’s energy and location within the atom. Here’s a breakdown of each number:
Principal Quantum Number (n)
- Determines the energy level of the electron. Larger values of n correspond to higher energy levels, further away from the nucleus.
- Allowed values: positive integers starting from 1 (n = 1, 2, 3, …).
- Example: Electrons in the innermost shell (1s) have n = 1, while those in the second shell (2s, 2p) have n = 2.
Azimuthal Quantum Number (l)
- Defines the sub-shell (or orbital type) the electron occupies within a principal energy level. Different sub-shells have distinct shapes and capacities.
- Allowed values: 0 ≤ l ≤ (n – 1). So, l = 0 for s orbitals, l = 1 for p orbitals, and so on.
- Example: In the second energy level (n = 2), an electron with l = 0 occupies the 2s sub-shell (spherical), while one with l = 1 occupies a 2p sub-shell (dumbbell-shaped).
Magnetic Quantum Number (ml)
- Specifies the orientation of the electron’s orbital within a subshell. Different ml values represent different possible orientations in space.
- Allowed values: For example, a p orbital (l = 1) can have ml = -1, 0, or 1, corresponding to three different spatial orientations.
- Example: Electrons in a p orbital with ml = 0 lie along the z-axis, while those with ml = ±1 lie in the x-y plane at different angles.
Electron Spin Quantum Number (ms)
- Describes the intrinsic spin of the electron, a fundamental property unrelated to its motion.
- Allowed values: ±1/2. Represents two possible spin states, often visualized as “up” and “down”.
- Example: Two electrons in the same orbital must have opposite spin states (+1/2 and -1/2) according to the Pauli Exclusion Principle.
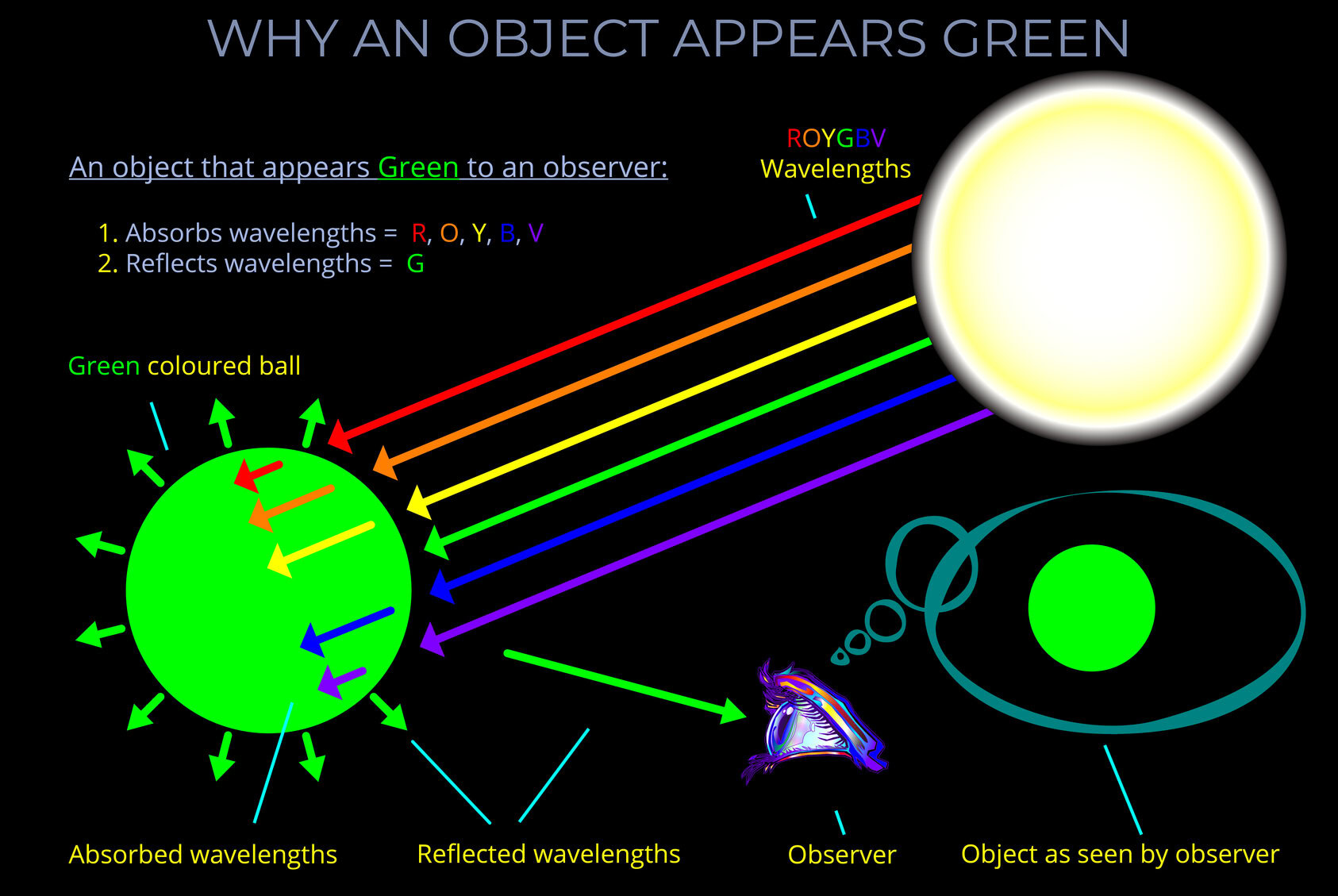
Related diagrams
Each diagram below can be viewed on its own page with a full explanation.