Electric and magnetic fields are two aspects of the same fundamental force, the electromagnetic force. The electromagnetic force is responsible for the attraction and repulsion between electrically charged particles, as well as for the propagation of light and other forms of electromagnetic radiation.
-
- First of all, let’s clarify the connection between force and field in this definition.
- A force is a push or pull on an object. A field is a region of space in which a force acts on objects.
- In the case of the electromagnetic force, the electric field is the region of space in which electric forces act on charged particles, and the magnetic field is the region of space in which magnetic forces act on moving charged particles.
- In other words, electric and magnetic fields are the regions in space where the electromagnetic force pushes and pulls microscopic objects such as electrons and macroscopic objects such as magnets or metal filings.
- First of all, let’s clarify the connection between force and field in this definition.
Interconnection of Electric and Magnetic Fields
-
- Electric and magnetic fields are interconnected, and changes in one field can induce changes in the other.
- Electromagnetic waves are produced when electric and magnetic fields oscillate together.
- A change in an electric field induces a change in the magnetic field.
- A change in a magnetic field induces a change in the electric field.
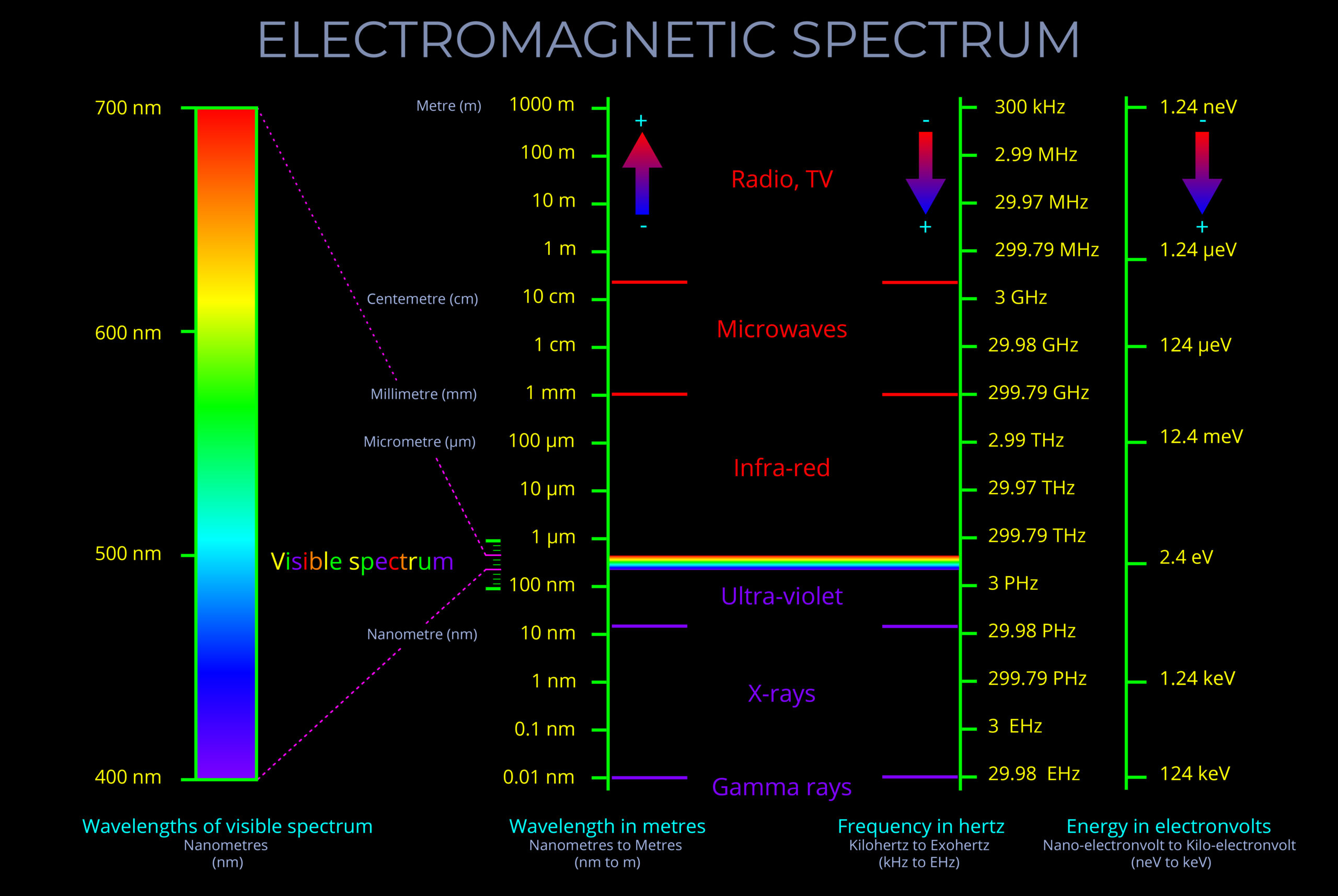
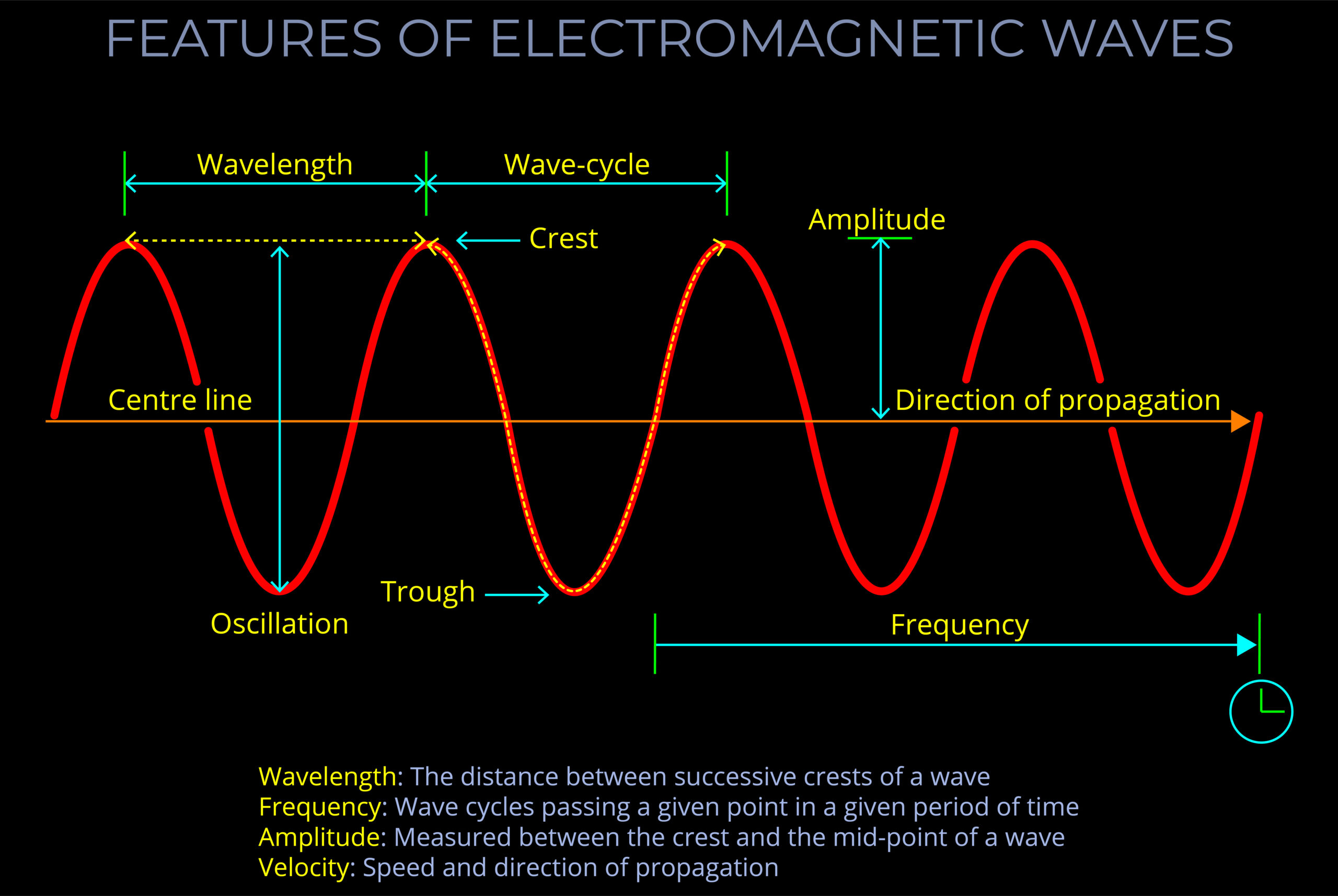
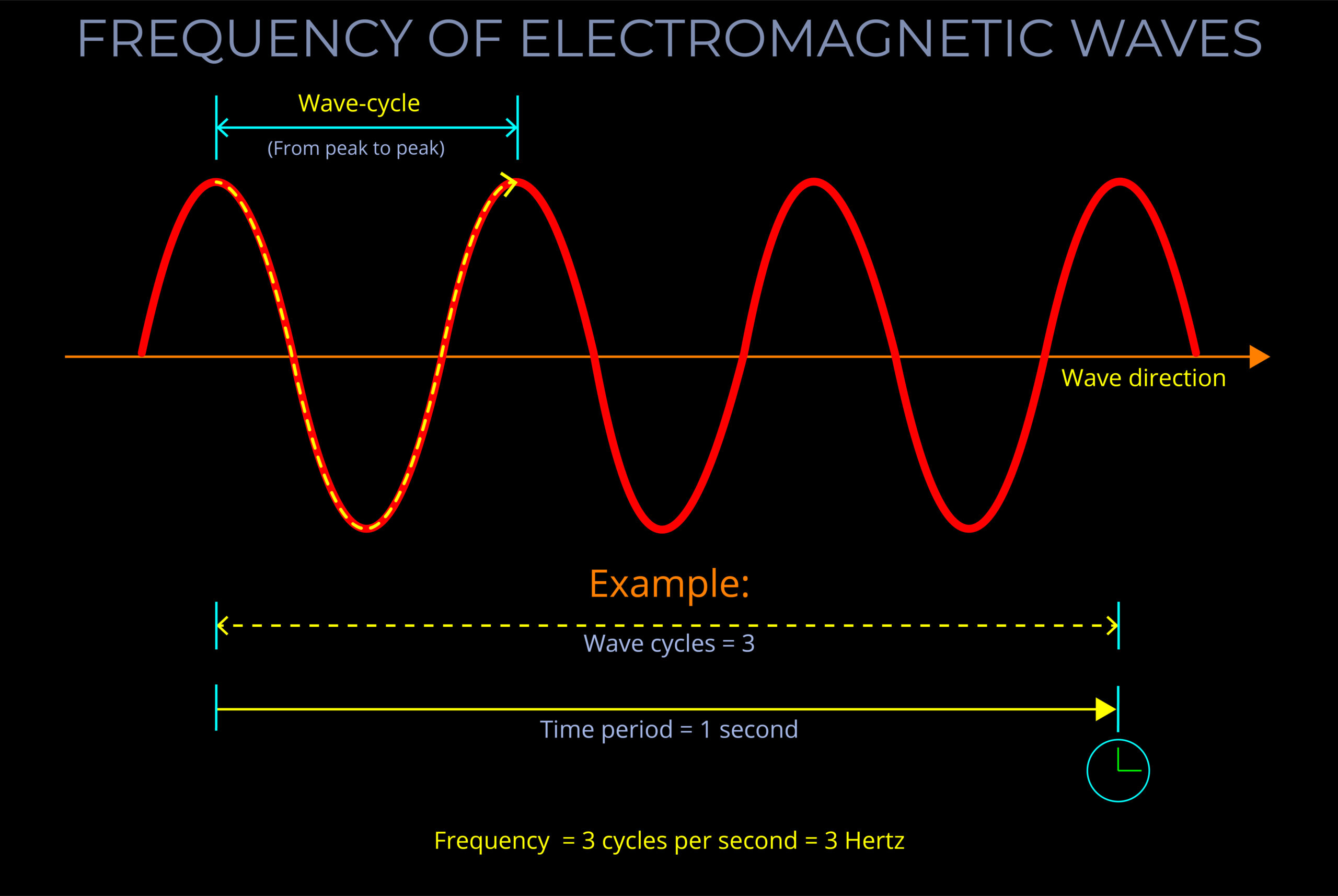
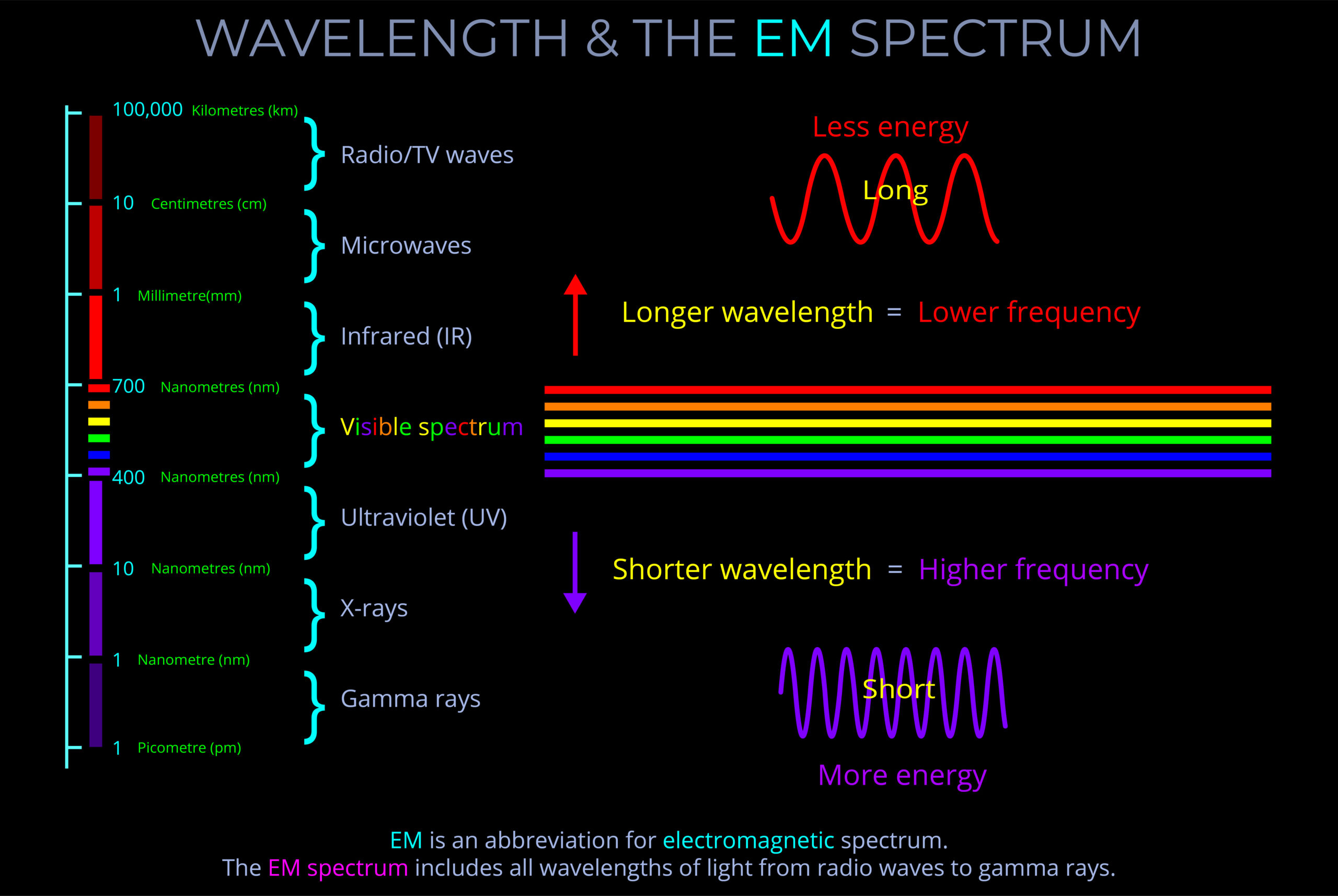
Characteristics of Electromagnetic Waves
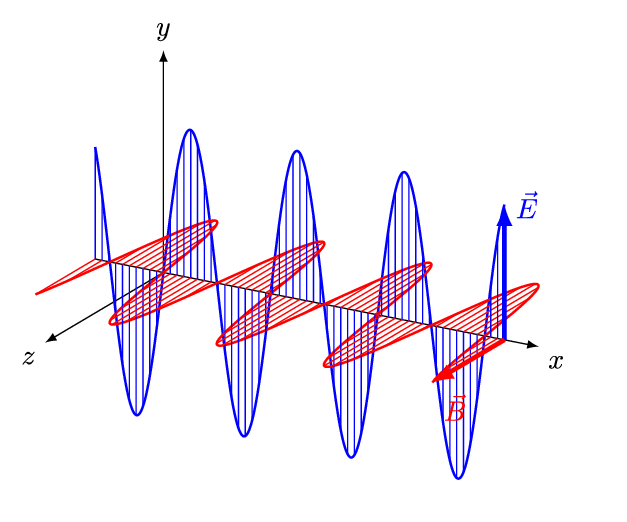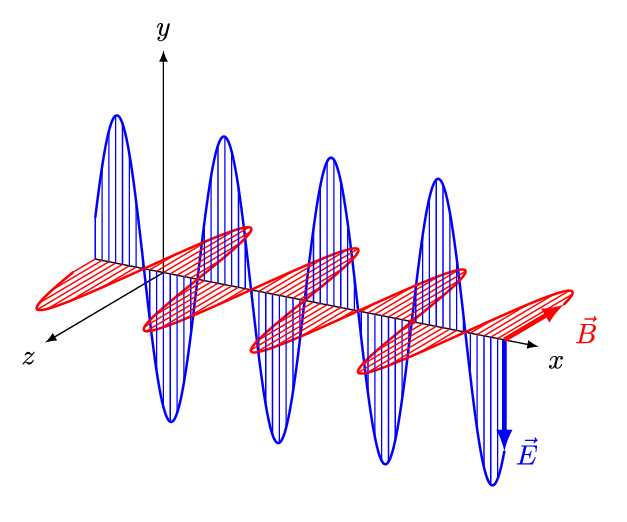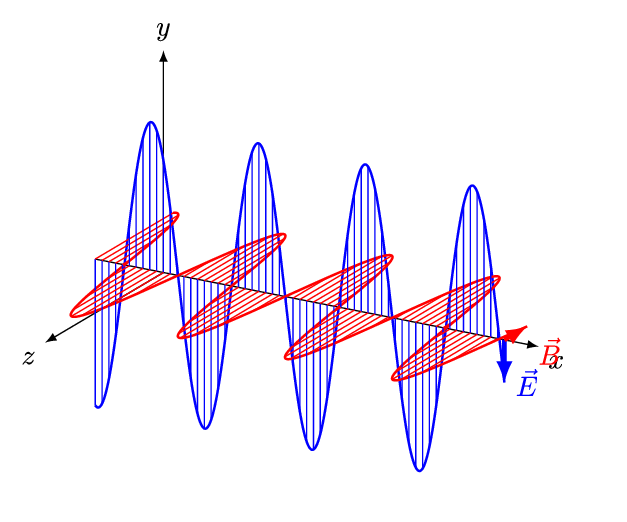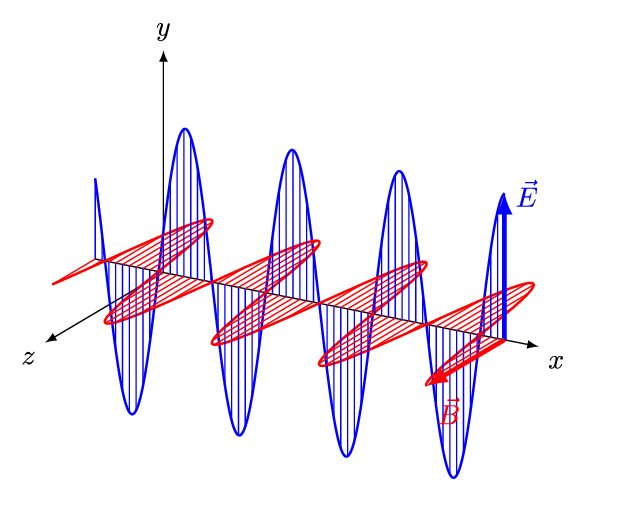
- An electromagnetic wave, travelling at the speed of light in a vacuum, is produced when either the voltage (charge) of an electric field changes or when the amperage (current) in a conductor changes. These changes in electric and magnetic fields result in synchronized oscillations, propagating at right angles to each other. This type of wave is often referred to as a transverse wave.
- The velocity at which electromagnetic waves propagate in a vacuum is the speed of light which is 299,792 kilometres per second.
Propagation of Electromagnetic Waves
- Once an electromagnetic wave radiates outward, it generally remains unaffected by external electric or magnetic fields. This is because electromagnetic waves are not composed of particles in the same way that matter is. They are more accurately described as disturbances in the electromagnetic field.
- There are exceptions to this rule:
- If an electromagnetic wave passes through an immensely strong magnetic field, it may experience slight deflection.
- Another exception is the deflection of electromagnetic waves by gravitational fields. However, gravitational deflection of light only takes place in the presence of objects like galaxies and black holes.
Nature of Electromagnetic Waves
- It’s important to note that electromagnetic waves do not require a medium to propagate through. This characteristic is related to the nature of photons.
- Photons are elementary particles that constitute electromagnetic radiation, meaning that electromagnetic radiation can be viewed as a stream of photons.
- While electromagnetic waves can travel through a vacuum, they can also interact with and be affected by matter when they pass through different materials.
- This interaction with matter forms the basis for various applications in optics, communications, and imaging, where electromagnetic waves interact with substances like glass, metals, or biological tissues.
Electromagnetic induction
-
-
- Whilst the material above focuses on the connection between electric and magnetic fields and light, let us quickly summarise their connection to the generation and utilization of electrical energy.
- In the most simple terms, electromagnetic induction is a way of converting mechanical energy into electrical energy and vice versa. by moving a magnet near a wire or changing the magnetic field around a wire.
- When a magnet is moved near a wire, it creates a changing magnetic field around the wire. This changing magnetic field induces an electric current in the wire. The direction and magnitude of the induced current depend on the direction and speed of the moving magnet, as well as the orientation of the wire.
- Electromagnetic induction can also be used to convert electrical energy into mechanical energy. This is achieved using motors. Motors use electromagnetic induction to generate a rotating force, which can then be used to power devices like fans, pumps, compressors, or electric vehicles.
-
- An electric field is created by a change in voltage (charge). The higher the voltage the stronger the field.
- Electric and magnetic fields are two aspects of the same fundamental force, the electromagnetic force. The electromagnetic force is responsible for the attraction and repulsion between electrically charged particles, as well as for the propagation of light and other forms of electromagnetic radiation.
- The connection between force and field in this definition can be summarized as:
- A force is a push or pull on an object. A field is a region of space in which a force acts on objects.
- In the case of the electromagnetic force, the electric field is the region of space in which electric forces act on charged particles, and the magnetic field is the region of space in which magnetic forces act on moving charged particles.
- In other words, electric and magnetic fields are the regions in space where the electromagnetic force pushes and pulls microscopic objects such as electrons and macroscopic objects such as magnets or metal filings.