The electrostatic force, also known as the Coulomb force, and the magnetic force, described by the Lorentz force equation, are distinct yet connected manifestations of the electromagnetic force.
- The electrostatic force is sometimes called the electric force. Both terms refer to the force that arises between charged particles. The word “electrostatic” emphasizes that the force is due to stationary (static) charges, while the word “electric” is a more general term that encompasses both static and moving charges.
- These two forces, electrostatic and magnetic articulate the behaviour of the electromagnetic field. They reveal that electric and magnetic fields are two facets of a unified electromagnetic field.
- The magnitude of the electrostatic force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
- The electrostatic force is the force between two electrically charged particles, regardless of whether they are moving or not.
- The magnetic force is the force between two moving electric charges, or between a magnetic field and a moving electric charge.
- The magnetic force is responsible for the attraction between oppositely charged magnets, such as a north pole and a south pole. It is also responsible for the repulsion between like-charged magnets, such as two north poles or two south poles.
- The magnetic force is responsible for the attraction between a magnet and a piece of iron. This is because iron is a ferromagnetic material, which means that it can be magnetized. When a magnet is brought near a piece of iron, the magnetic force of the magnet aligns the magnetic domains in the iron, causing the iron to become temporarily magnetized.
- The electrostatic force and the magnetic force are unified by Maxwell’s equations, which describe the behaviour of the electromagnetic field. Maxwell’s equations show that the electric and magnetic fields are two aspects of the same field and that they are related to each other.
- The electromagnetic force is one of the four fundamental forces of nature. The other three fundamental forces are the strong nuclear force, the weak nuclear force, and gravity. The electromagnetic force is the strongest of the four fundamental forces at the atomic and macroscopic levels.
References
- 1
- 2
Summary
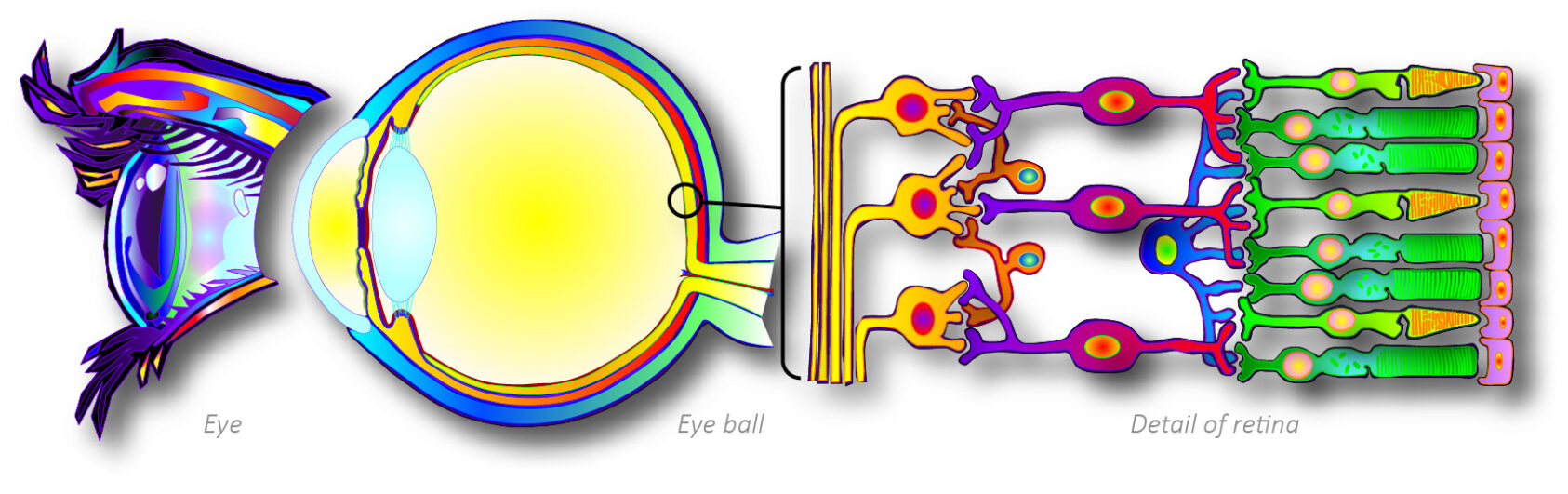 Cross-section of the human eyeball
Cross-section of the human eyeball