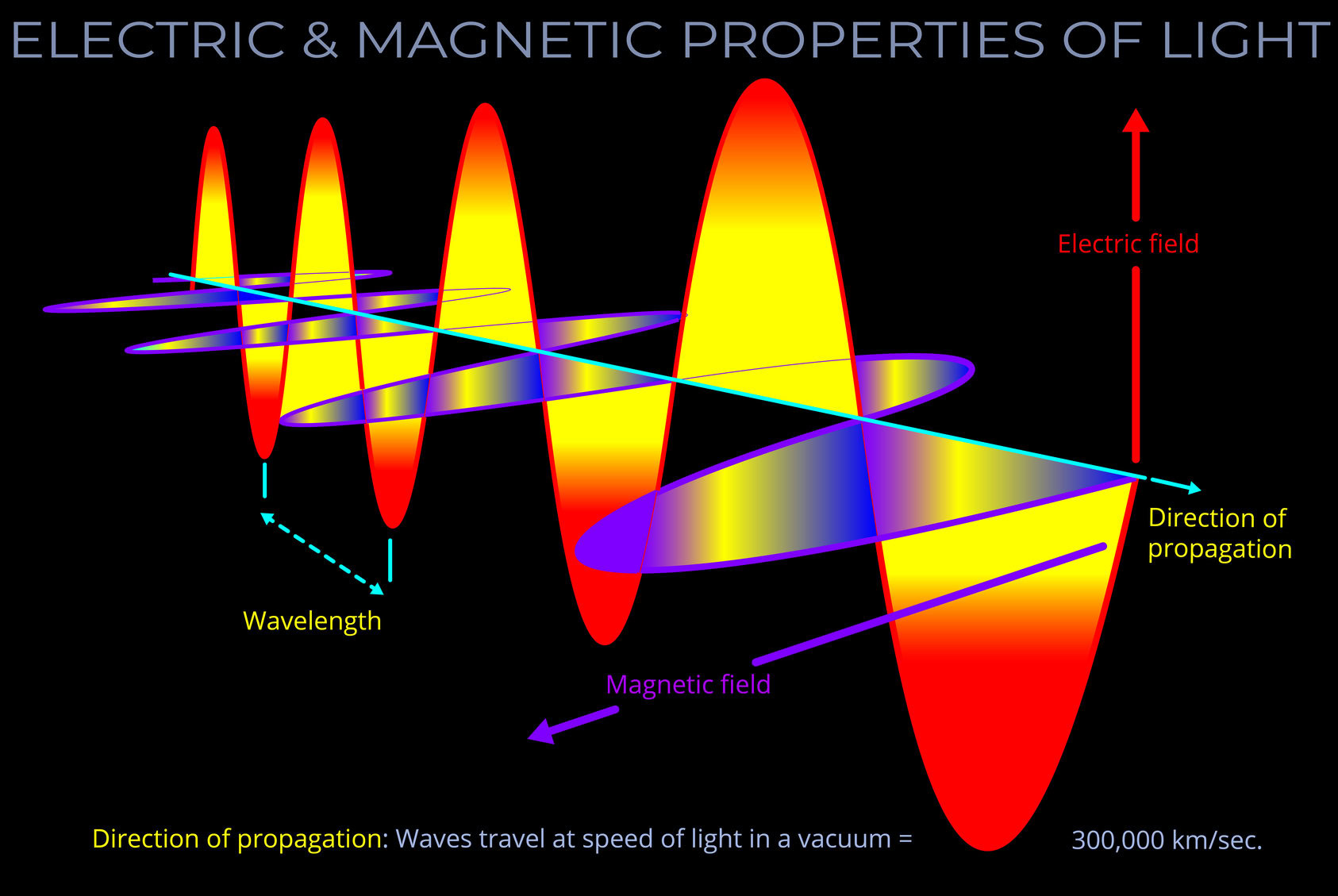
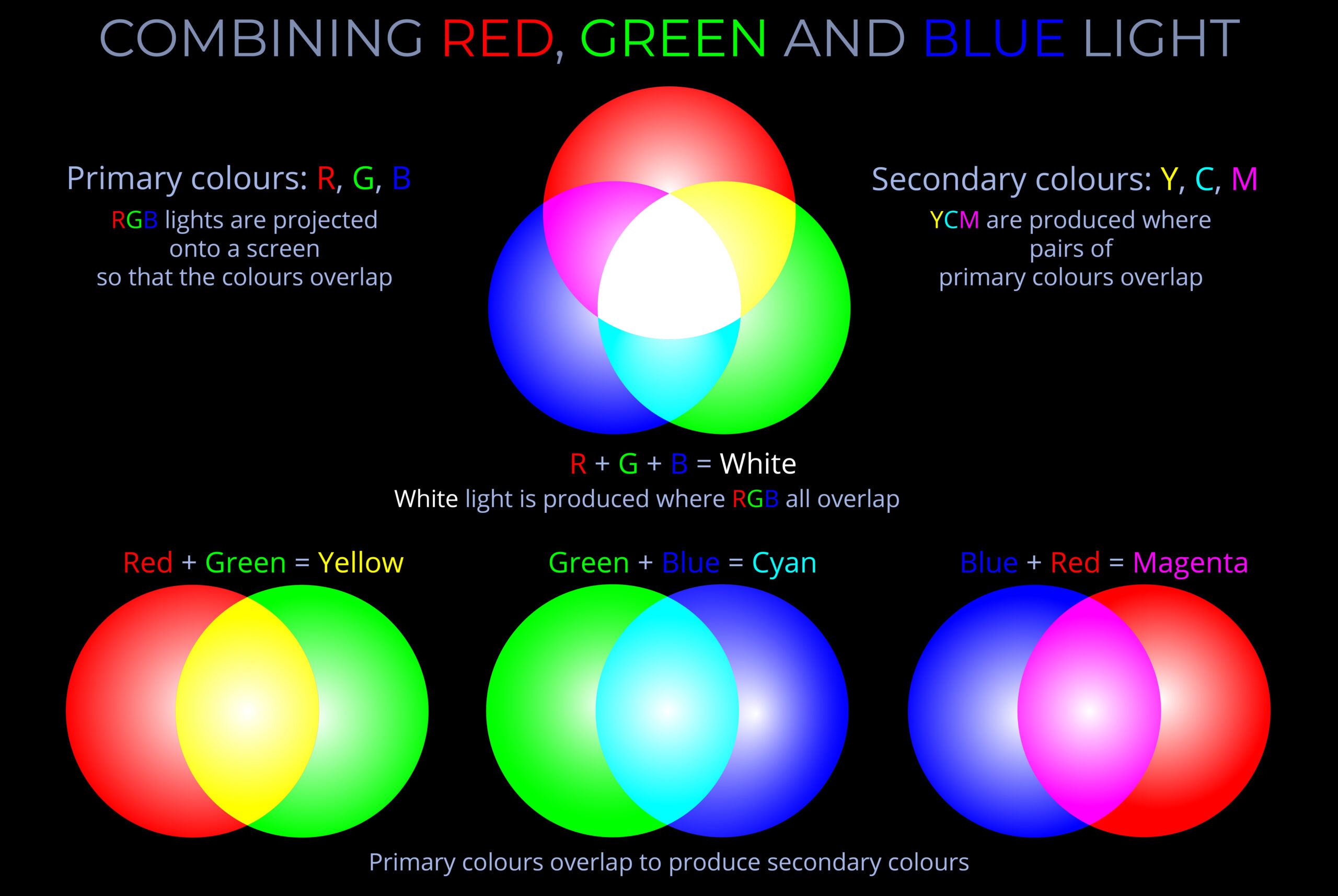
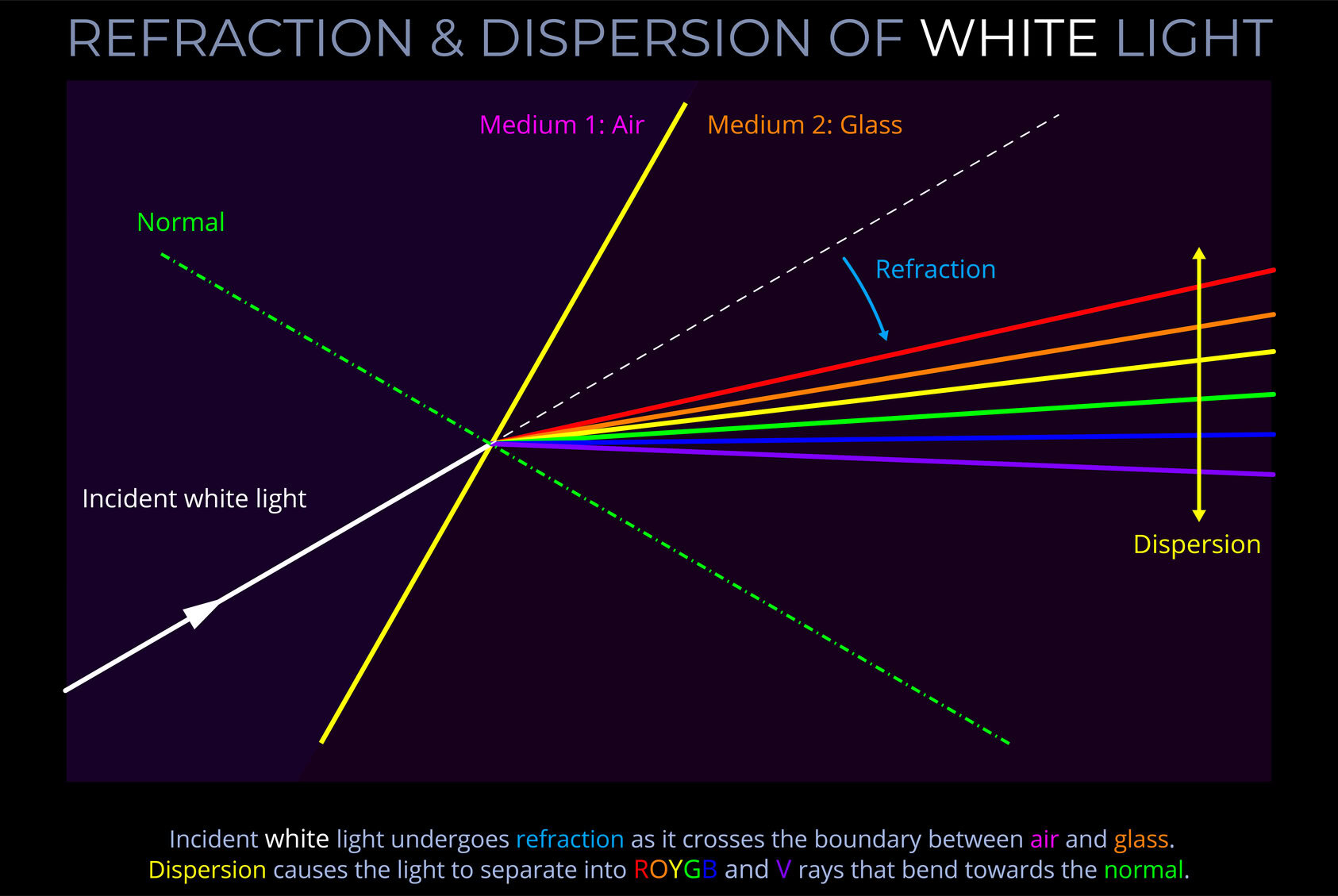
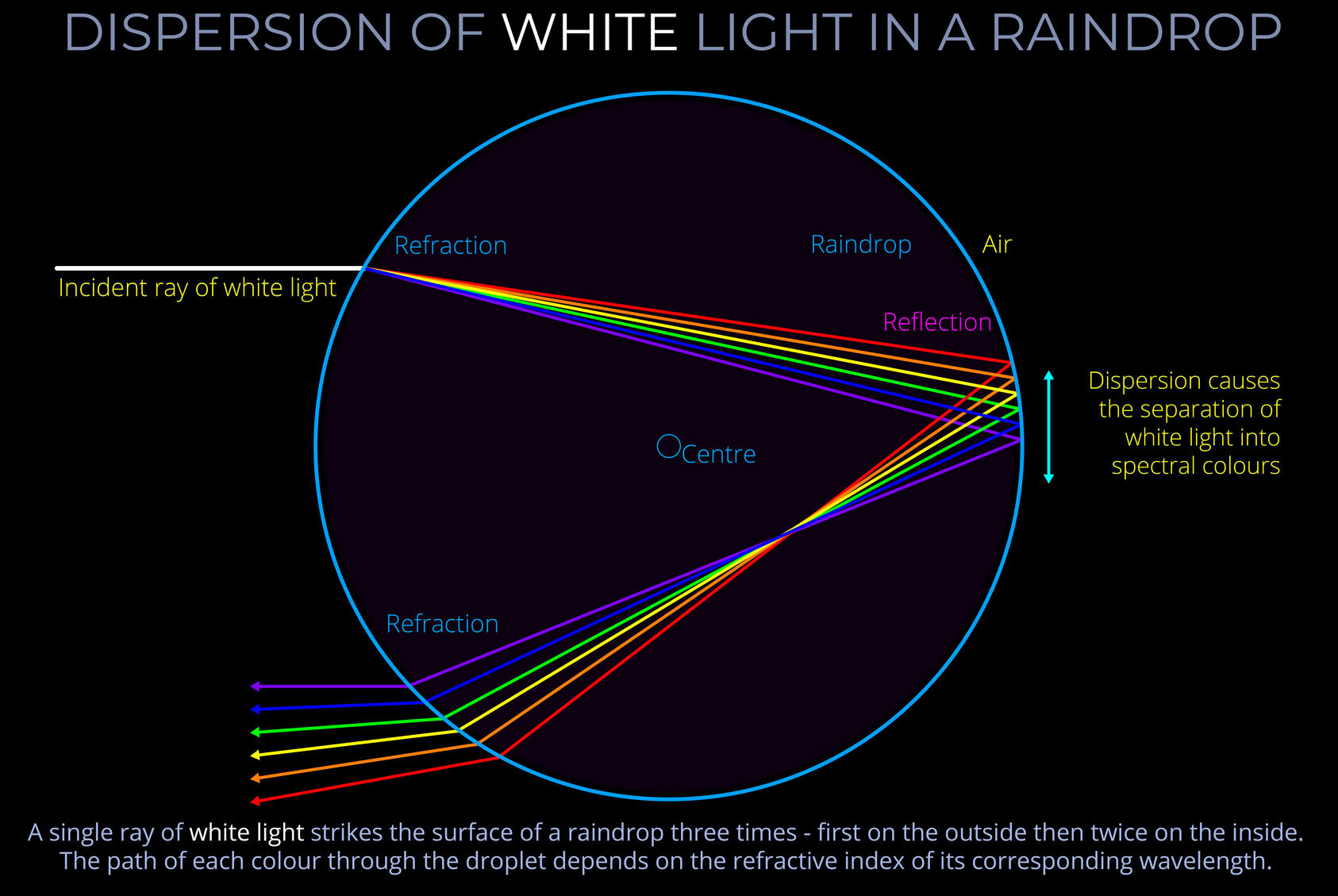
A light source is any object that emits electromagnetic radiation within the visible spectrum or other areas of the electromagnetic spectrum.
- A light source is a natural or man-made object that emits one or more wavelengths of light.
- Natural light sources encompass a vast spectrum of electromagnetic radiation, ranging from long, low-energy radio waves to powerful, high-energy bursts of gamma rays.
- An artificial light source is an object or device that creates visible light without relying on natural processes such as sunlight, bioluminescence, or atmospheric phenomena. It utilizes various mechanisms to convert other forms of energy into visible light.
NATURAL SOURCES OF LIGHT
A list of natural light sources might include:
- Solar Radiation: This is the most abundant natural light source, originating from the sun’s nuclear fusion process. This light travels to Earth in various wavelengths, including visible light, ultraviolet (UV) light, and infrared (IR) radiation.
- Bioluminescence: Bioluminescence involves living organisms such as fireflies, deep-sea creatures, and some fungi emitting light through chemical reactions.
- Triboluminescence: This less common form of light emission occurs when certain materials, like sugar or quartz, are subjected to friction or stress, causing them to glow briefly.
- Chemiluminescence: This chemical reaction produces light as a byproduct. It is seen in glow sticks, fireflies, and certain biological processes. The chemical reaction excites the electrons within these molecules, causing them to jump to higher energy levels. When they return to their ground state, they release energy in the form of light.
- Atmospheric phenomena: Lightning, auroras, and airglow (faint nighttime glow) are examples of light produced by interactions between atmospheric gases, charged particles, and electromagnetic fields. These processes involve various mechanisms like electron excitation, recombination, and collisions.
ARTIFICIAL SOURCES OF LIGHT
A list of natural artificial sources might include:
- Incandescent light: This traditional lighting method relies on heating a filament (usually tungsten) to high temperatures until it glows, emitting a warm, yellowish light. Incandescent bulbs are less efficient than many other options.
- Fluorescent light: These tube-shaped lamps use fluorescent coatings that convert ultraviolet radiation from a mercury vapour discharge into visible light. They come in various colour temperatures and offer higher efficiency than incandescent bulbs.
- LED lighting: Light-emitting diodes (LEDs) are popular due to their high efficiency and long lifespan. They convert electrical energy directly into light with minimal heat generation, and their colour temperature can be tailored to various applications.
- Gas-discharge lamps: These encompass high-intensity discharge (HID) lamps, sodium-vapour lamps, and metal halide lamps. They use electrical discharges in different gases to create high-intensity lighting with colour spectra that suit applications such as street lighting and stadiums.
ABOUT NATURAL & ARTIFICIAL LIGHT: SUB-ATOMIC PROCESSES
Here is a list of key sub-atomic processes involved in the generation of both natural and artificial light, categorized by the nature of the process:
Electron Transitions
- Atomic emission: Excited electrons in atoms lose energy by dropping to lower energy levels, emitting photons (light) with specific wavelengths. (Natural: Stellar light, Auroras; Artificial: Fluorescent lamps, Gas discharge lamps)
- Recombination: Electrons recombine with positive ions (missing electrons), releasing energy as photons. (Natural: Nebulae; Artificial: LEDs, Semiconductor lasers)
Radiative Decay
- Nuclear decay: Certain radioactive decay processes (alpha decay, gamma decay) involve the direct emission of photons from the nucleus. (Natural: Radioactive materials; Artificial: Nuclear reactors, Radioisotope tracers)
- Annihilation: Particle-antiparticle collisions result in complete mass conversion to energy, often releasing high-energy photons (gamma rays). (Natural: Cosmic ray interactions; Artificial: Positron emission tomography (PET) scans)
Collisional Processes
- Bremsstrahlung: Charged particles interacting with other charged particles or strong electric fields decelerate, emitting photons. (Natural: Stellar interiors, Black hole accretion disks; Artificial: X-ray machines)
- Synchrotron radiation: Charged particles moving at high speeds in magnetic fields experience acceleration and emit characteristic light spectra. (Natural: Pulsars, Magnetars; Artificial: Synchrotrons, Particle accelerators)
Other Processes
- Cherenkov radiation: Charged particles travelling faster than light in a medium emit a faint bluish glow due to this specific radiation. (Natural: Cosmic rays in water; Artificial: Cherenkov detectors)
- Piezoluminescence: Certain materials emit light under pressure due to electron transitions induced by mechanical stress. (Natural: Quartz crystals; Artificial: Ultrasonic cleaning devices)
- Sonoluminescence: Cavitation bubbles collapsing in liquids can produce brief flashes of light due to various mechanisms (exact processes still debated). (Natural: Shrimp snapping claws; Artificial: Sonoluminescence research)
Additionally
- Chemical reactions: Some chemical reactions directly convert chemical energy into light through specific mechanisms (chemiluminescence) involving excited molecules. (Natural: Fireflies, Deep-sea creatures; Artificial: Glow sticks)
- Plasma: Hot, ionized gases (plasmas) exhibit various processes leading to light emission, including recombination, Bremsstrahlung, and interactions with charged particles. (Natural: Stellar atmospheres, Lightning; Artificial: Plasma torches, Fusion reactors)
| Light sources | Emission mechanism | Description | Examples |
|---|---|---|---|
| LIGHT-EMITTING PROCESS | |||
| Luminescence | Light emission due to the excitation of electrons in a material. | Electrons within a material gain energy and then release light as they return to a lower energy state. | Bioelectroluminescence Electroluminescence Photoluminescence - Fluorescence - Phosphorescence Sonoluminescence Thermoluminescence |
| Blackbody radiation (Type of thermal radiation) | Electromagnetic radiation (including visible light) emitted by any object with a temperature above absolute zero. | Electromagnetic radiation (including visible light) emitted by any object with a temperature above absolute zero. | All objects above temperature of absolute zero. |
| Chemiluminescence | Light from natural and artificial chemical reactions. | Light from natural and artificial chemical reactions. | Bioluminescence Chemiluminescent reactions: - Luminol reactions - Ruthenium chemiluminescence |
| Nuclear reaction | Light emission as a byproduct of nuclear reactions (fusion or fission). | Light emitted as a byproduct of nuclear reactions. | Nuclear reactors Stars undergoing fusion |
| Thermal radiation | Light emission due to the thermal excitation of atoms and molecules at high temperatures. | Light emission due to the thermal excitation of atoms and molecules. | Sun Stars Incandescent light bulbs |
| Triboluminescence | Light emission due to mechanical stress applied to a material. | Light emission due to the mechanical stress applied to a material, causing the movement of electric charges and subsequent light emission. | Sugar crystals cracking Adhesive tape peeling Quartz crystals fracturing. |
| Natural light source | |||
| Fireflies Deep-sea creatures Glowing mushrooms | Bioluminescence | Light emission from biological organisms. | Involves the luciferase enzyme. |
| Sun Stars | Nuclear Fusion | Light emission as a byproduct of nuclear fusion reactions in stars. | Electromagnetic spectrum (visible light, infrared, ultraviolet). |
| Fire Candles | Thermal radiation | Light emission due to the thermal excitation of atoms and molecules during the combustion of a fuel source. | Burning of a fuel source, releasing heat and light. |
| Artificial light source | |||
| Fluorescent lights Highlighters Safety vests | Chemiluminescence | Light emission from chemical reactions. | Fluorescence (absorption and re-emission of light). |
| Glow sticks Emergency signs | Chemiluminescence | Light emission due to phosphorescence - a type of chemiluminescence. | A type of chemiluminescence where light emission is delayed after the initial excitation. |
| Glow sticks Light sticks | Chemiluminescence | Chemiluminescence | Light emission from a chemical reaction that does not involve combustion. |
| Tungsten light bulbs Toasters | Thermal radiation | Heated filament radiates light and heat. | Light emission from a hot filament. |
| Fluorescent lamps LED lights | Electroluminescence | Excitation of atoms by electric current. | Light emission when electric current excites atoms in a material. |
| Neon signs | Electrical Discharge | Discharge of electricity through gas. | Light emission when electricity flows through a gas. |
| Sugar crystals cracking Pressure-sensitive adhesives | Triboluminescence | Light emission from friction or pressure. | Light emission due to mechanical forces. |
| Fluorescent paint Highlighters Safety vests | Photoluminescence | Absorption and subsequent re-emission of light at a lower energy. | Absorption and re-emission of light. |
Light Sources: Mechanism, examples, and everyday applications
Footnote: Cerenkov radiation and Synchrotron radiation are not included in the table because they are not conventionally classified as light sources.
References
- https://en.wikipedia.org/wiki/Light#Light_sources
Here are some Wikipedia links to less familiar sources of light:
- Bioluminescence: Bioluminescence is the production and emission of light by living organisms.
- Bremsstrahlung: Bremsstrahlung from German bremsen ‘to brake’, and Strahlung ‘radiation’) is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into radiation (i.e., photons). The term is also used to refer to the process of producing the radiation. Bremsstrahlung has a continuous spectrum, which becomes more intense and whose peak intensity shifts toward higher frequencies as the change of the energy of the decelerated particles increases.
- Cherenkov radiation: Cherenkov radiation is electromagnetic radiation emitted when a charged particle (such as an electron) passes through a dielectric medium (such as distilled water) at a speed greater than the phase velocity of light (speed of propagation of a wavefront in a medium) in that medium.
- Electroluminescence: Electroluminescence (EL) is an optical and electrical phenomenon, in which a material emits light in response to the passage of an electric current or a strong electric field.
- Particle Collisions: High-energy photons (light) are produced during particle collisions in particle accelerators. A particle accelerator is a machine that uses electromagnetic fields to propel charged particles to very high speeds and energies and to contain them in well-defined beams.
- Particle–antiparticle annihilation: Particle–antiparticle pairs can annihilate each other, producing photons. Since the charges of the particle and antiparticle are opposite, total charge is conserved. For example, the positrons produced in natural radioactive decay quickly annihilate themselves with electrons, producing pairs of gamma rays, a process exploited in positron emission tomography.
- Scintillation: In condensed matter physics, scintillation is the process where a material, called a scintillator, emits ultraviolet or visible light under excitation from high energy photons such as X-rays or gamma rays or energetic particles such as electrons, alpha particles, neutrons, or ions.
- Sonoluminescence: Sonoluminescence is the emission of light from imploding bubbles in a liquid when excited by sound.
- Synchrotron Radiation: High-energy light emitted by charged particles accelerated in synchrotrons.
- Radioactive decay: Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha, beta, and gamma decay. The weak force is the mechanism that is responsible for beta decay, while the other two are governed by electromagnetism and the weak nuclear force.
- Triboluminescence: Triboluminescence is a phenomenon in which light is generated when a material is mechanically pulled apart, ripped, scratched, crushed, or rubbed (see tribology). The phenomenon is not fully understood but appears to be caused by the separation and reunification of static electric charges.