The spectral power distribution (SPD) provides a detailed profile of the light emitted or reflected by a source across the visible spectrum, typically represented as a graph where the x-axis shows the wavelength (or frequency) and the y-axis shows the intensity or power at each wavelength.
- Spectral power distribution is usually measured with a spectroscope. These instruments break down the light into its constituent wavelengths, allowing for precise analysis of the light’s spectral composition. This helps with understanding the exact colour of a light source or how it interacts with materials.
- SPD is critical in defining colour perception. The way the human eye perceives colour is heavily influenced by the distribution of power at various wavelengths, as different combinations of wavelengths will stimulate the cones in our retinas to varying degrees, resulting in a specific colour experience.
- SPD helps in identifying and comparing light sources. Different light sources, such as sunlight, LED lamps, or incandescent bulbs, have distinct SPDs. For instance, sunlight has a broad, continuous spectrum, while LEDs or fluorescents often have spikes at certain wavelengths, affecting how we perceive colour under these lights.
- SPD plays a key role in material appearance. When light reflects off a surface, the spectral power distribution of the reflected light reveals how different wavelengths are absorbed or reflected by the material, influencing the material’s colour and brightness.
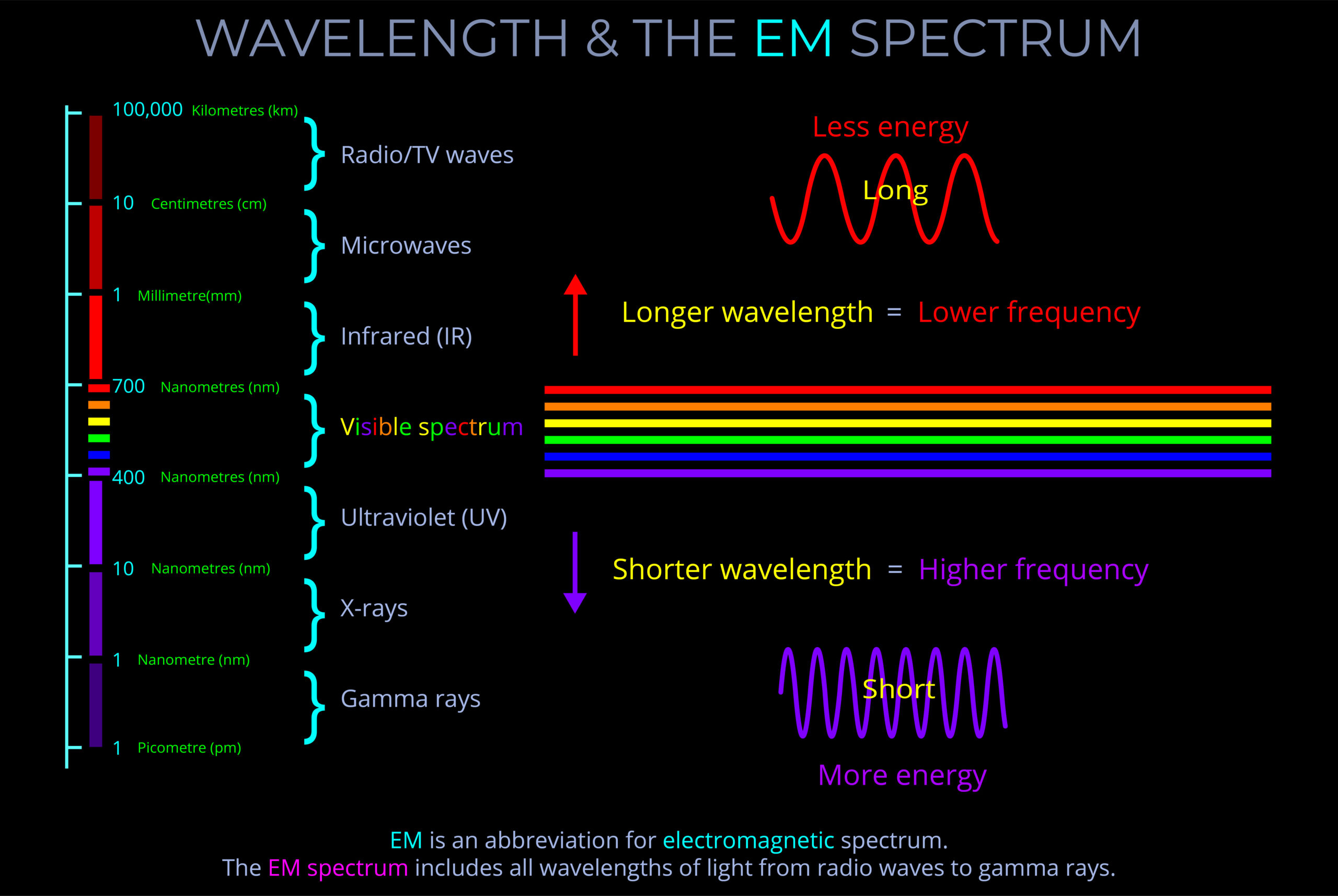
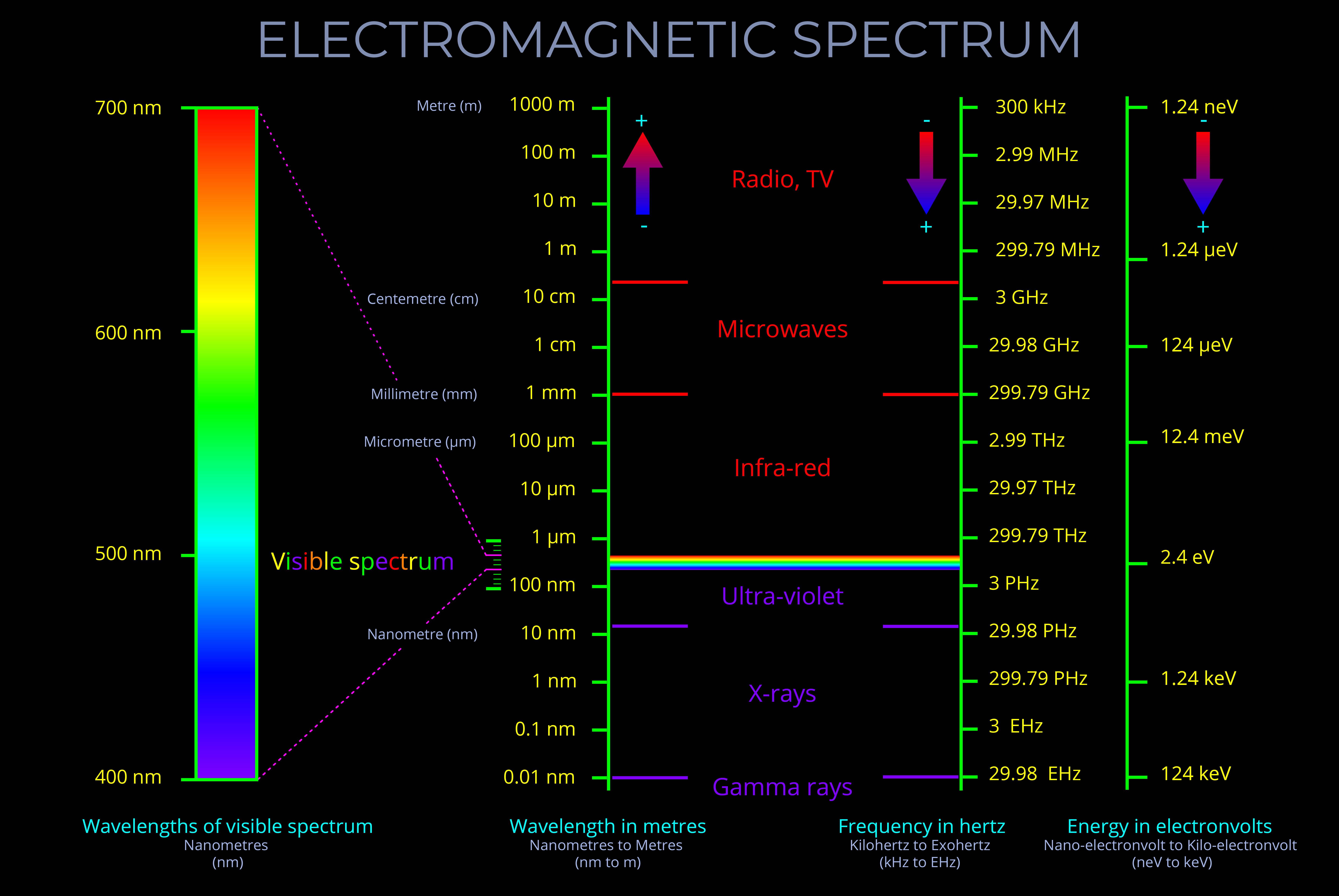
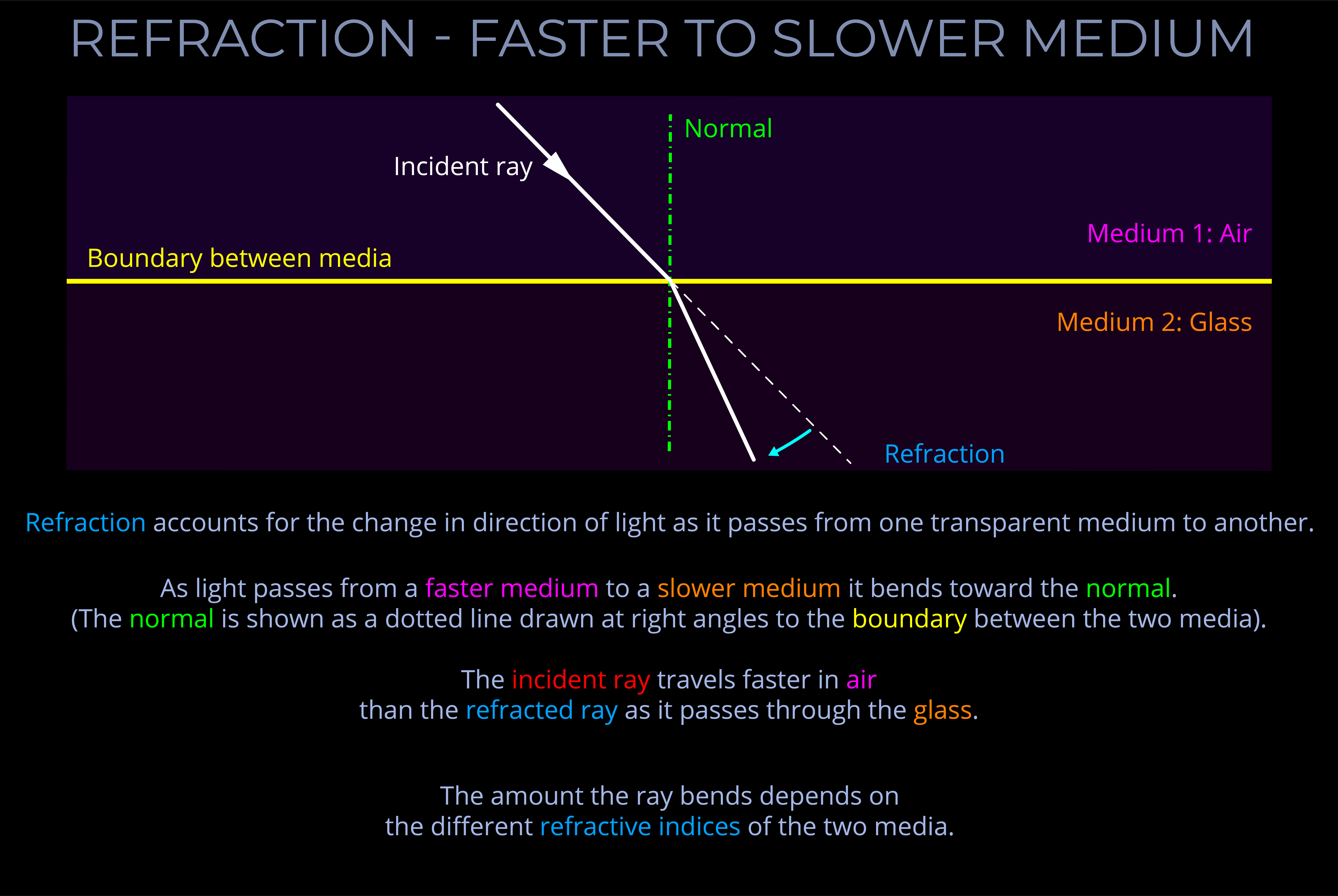
The visible spectrum refers to the range of colours the human eye can perceive, typically seen when light is refracted through a prism, water droplets, or similar mediums. It spans wavelengths from approximately 380 nm (violet) to 700 nm (red), with each wavelength corresponding to a specific colour, from violet through blue, green, yellow, and red.
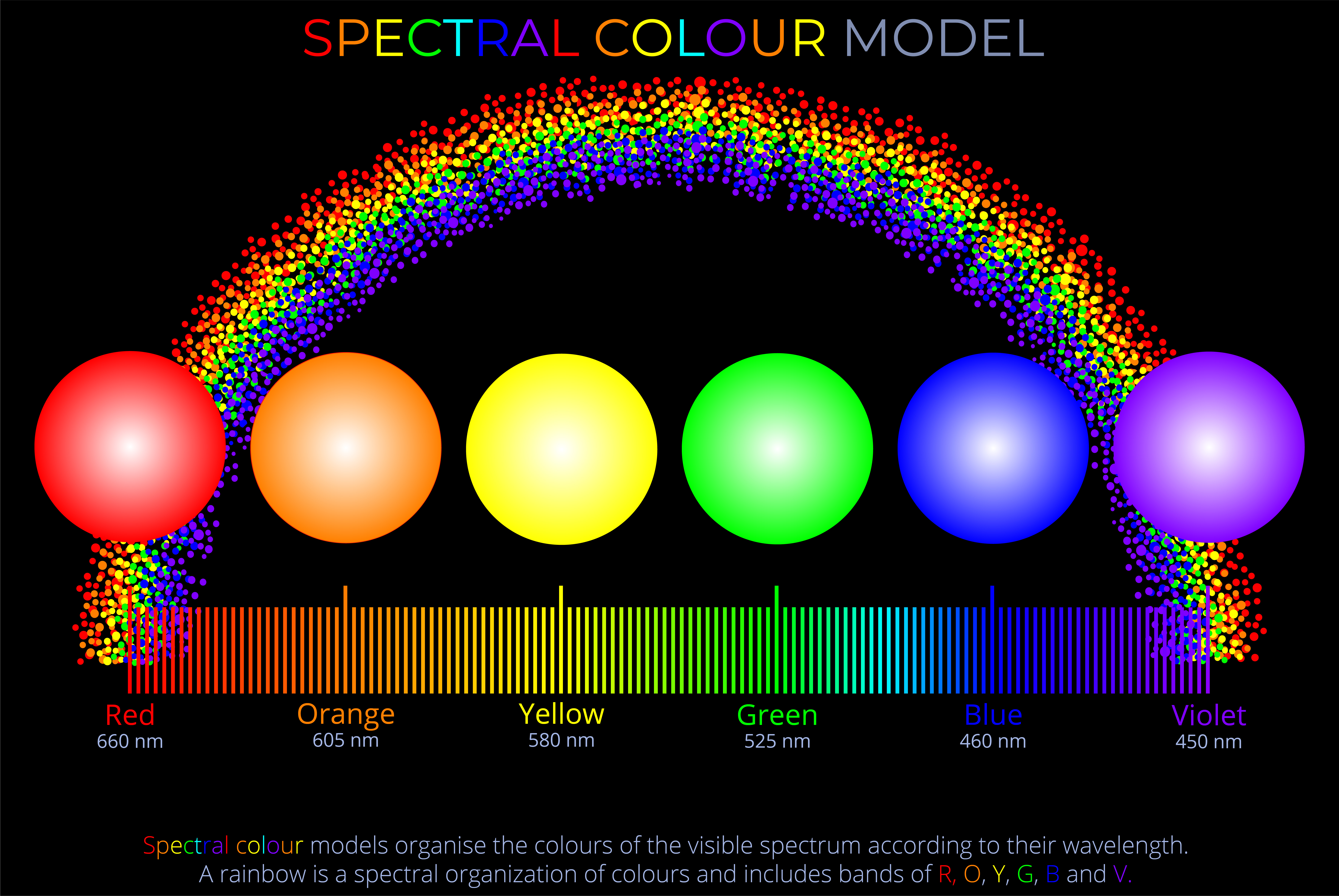
- The visible spectrum consists of a continuous distribution of colours, formed by a range of wavelengths rather than distinct, separate bands. While we commonly refer to colours like red, green, and violet, the transitions between them are gradual, with no sharp boundaries.
- A diagram of the visible spectrum typically displays this continuous range as a linear scale, arranged by wavelength, with red at the longer wavelength end (around 700 nm) and violet at the shorter wavelength end (around 380 nm). This kind of diagram allows us to see the full gradation of colours the human eye can perceive.
- The visible spectrum is naturally produced when light is refracted through a prism, raindrops, or similar mediums, splitting the light into its component wavelengths. This process of separating light is known as dispersion. The resulting diagram, often called a spectrum, visually represents the distribution of spectral colours as a smooth, elongated band from red to violet, enabling us to observe the gradual transitions between colours.
- Although the spectrum contains an infinite number of colours due to its continuous nature, most diagrams illustrate a limited number of distinguishable colours between red, orange, yellow, green, blue, indigo, and violet.
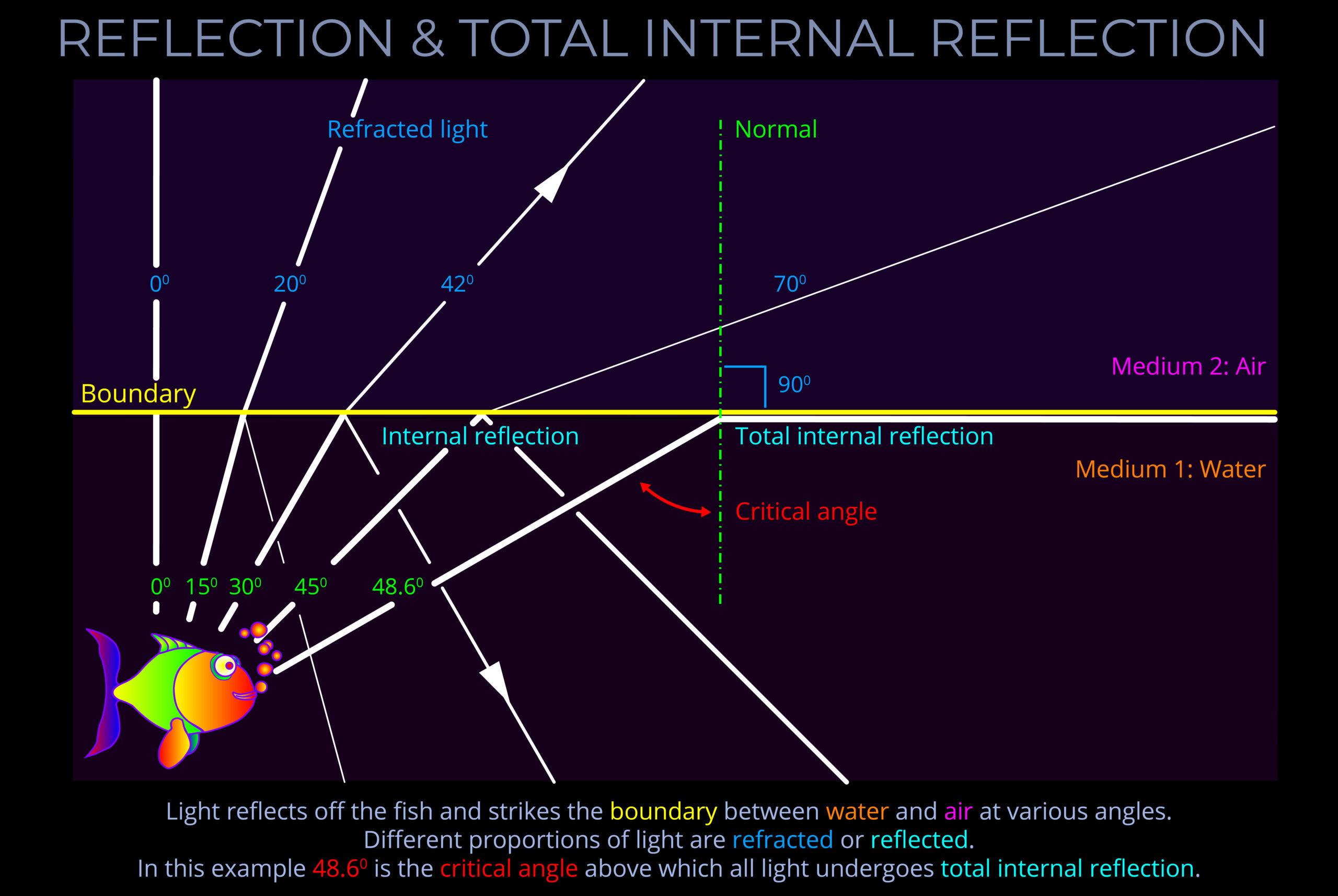
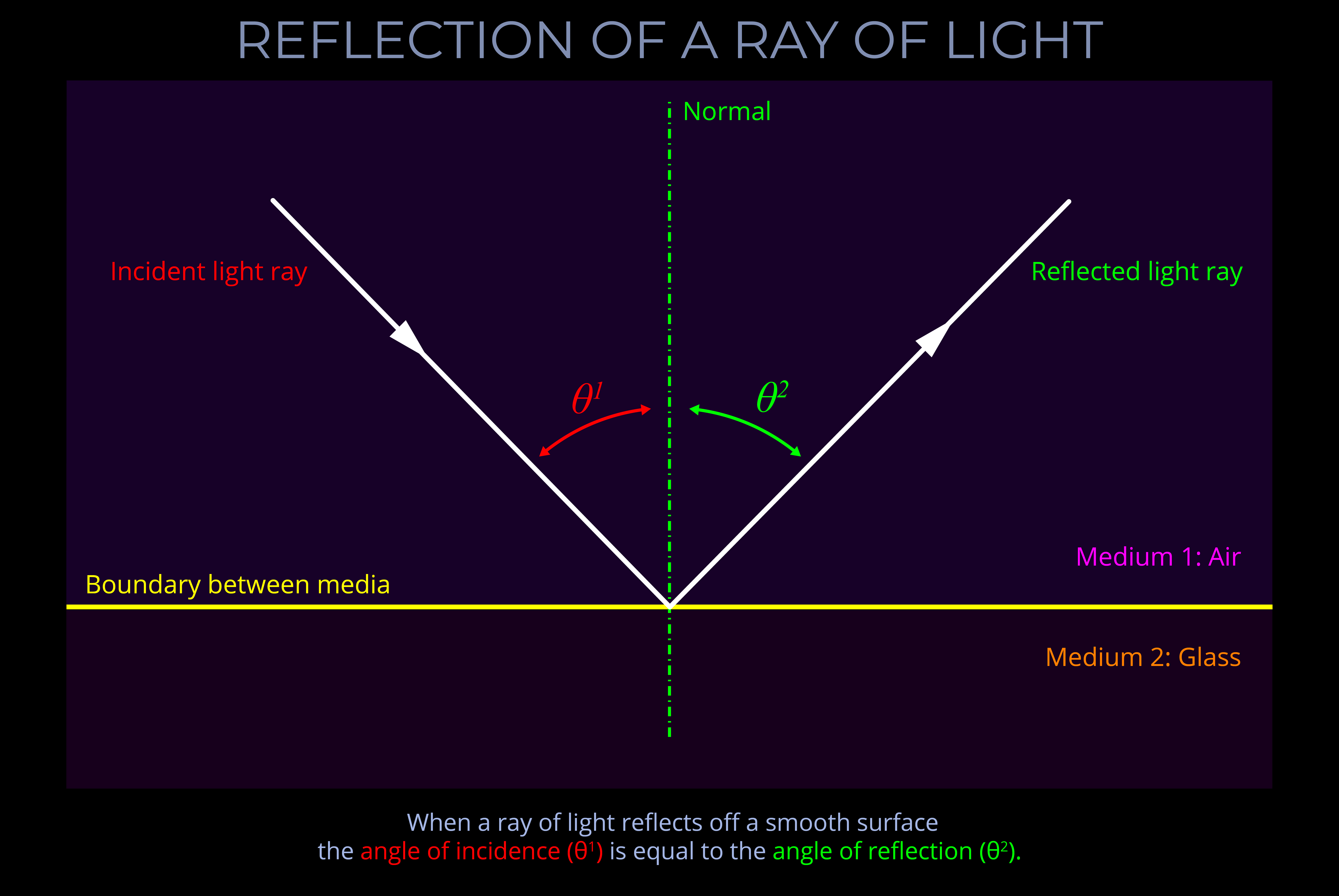
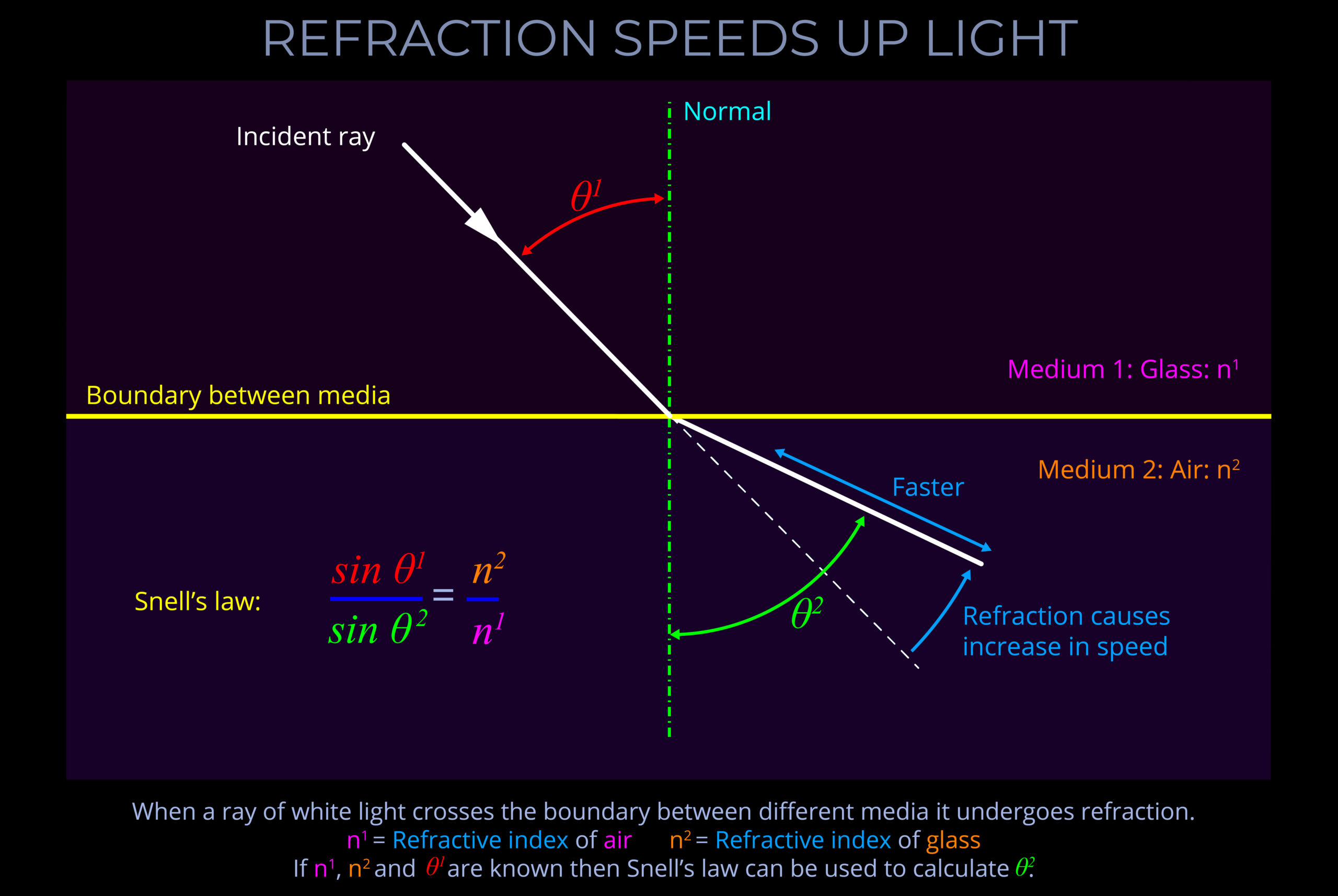
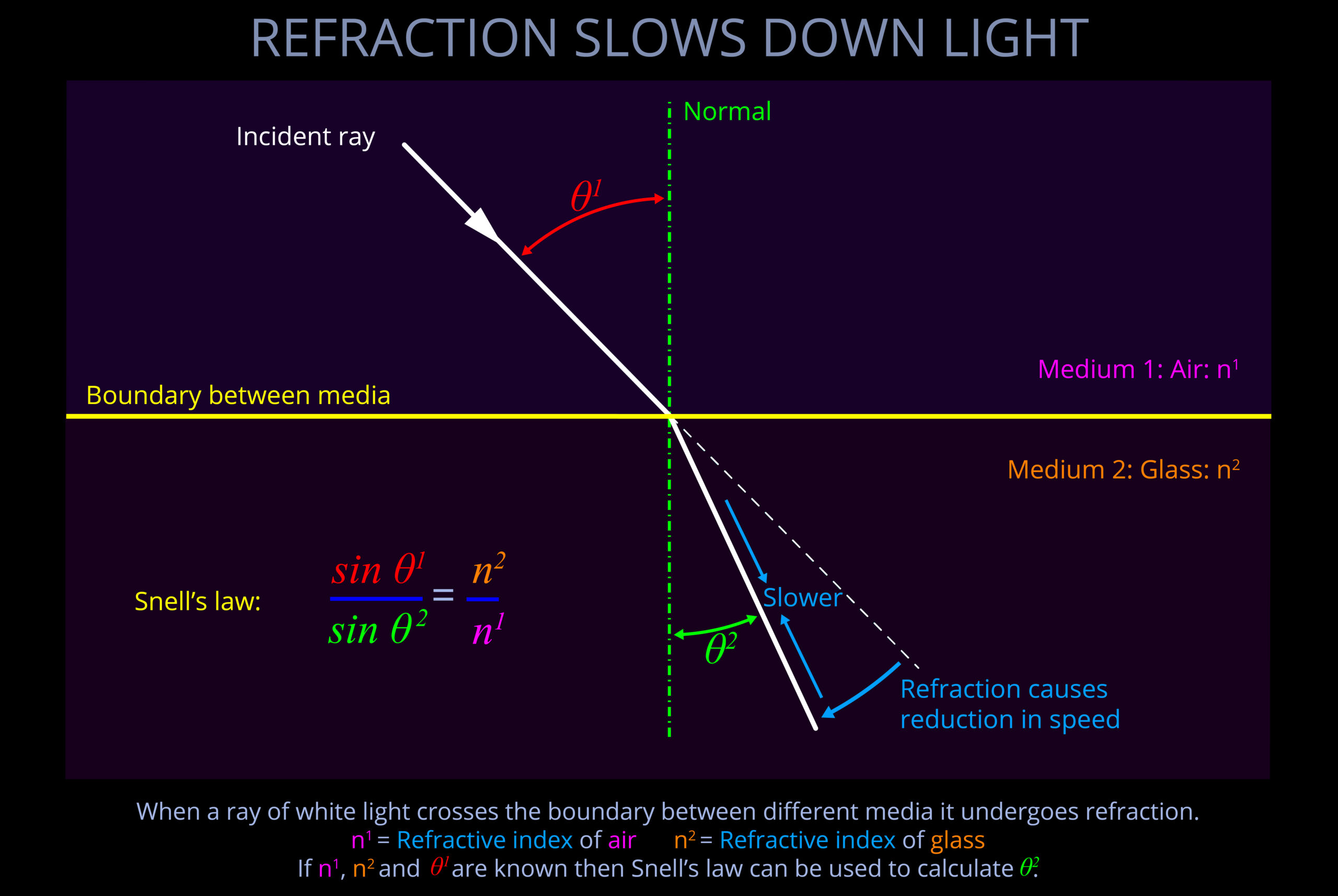
Objects with smooth surfaces produce specular (mirror-like) reflections because light reflects off their surfaces at consistent angles.
- All objects obey the law of reflection on a microscopic level.
- If the irregularities on the surface of an object are smaller than the wavelengths of incident light then reflected light travels away from the surface at consistent angles.
- When an observer looks at specular reflections (regular reflections), they see mirror-like images on the surface of an object.
About diffuse reflection
- If the irregularities on the surface of an object are larger than the wavelengths of the incident light, light reflects in all directions and produces diffuse reflections.
- A diffuse reflection is easily distinguished from the mirror-like qualities of a specular reflection.
- Diffuse reflection is responsible for the way we perceive the colours and textures of objects.
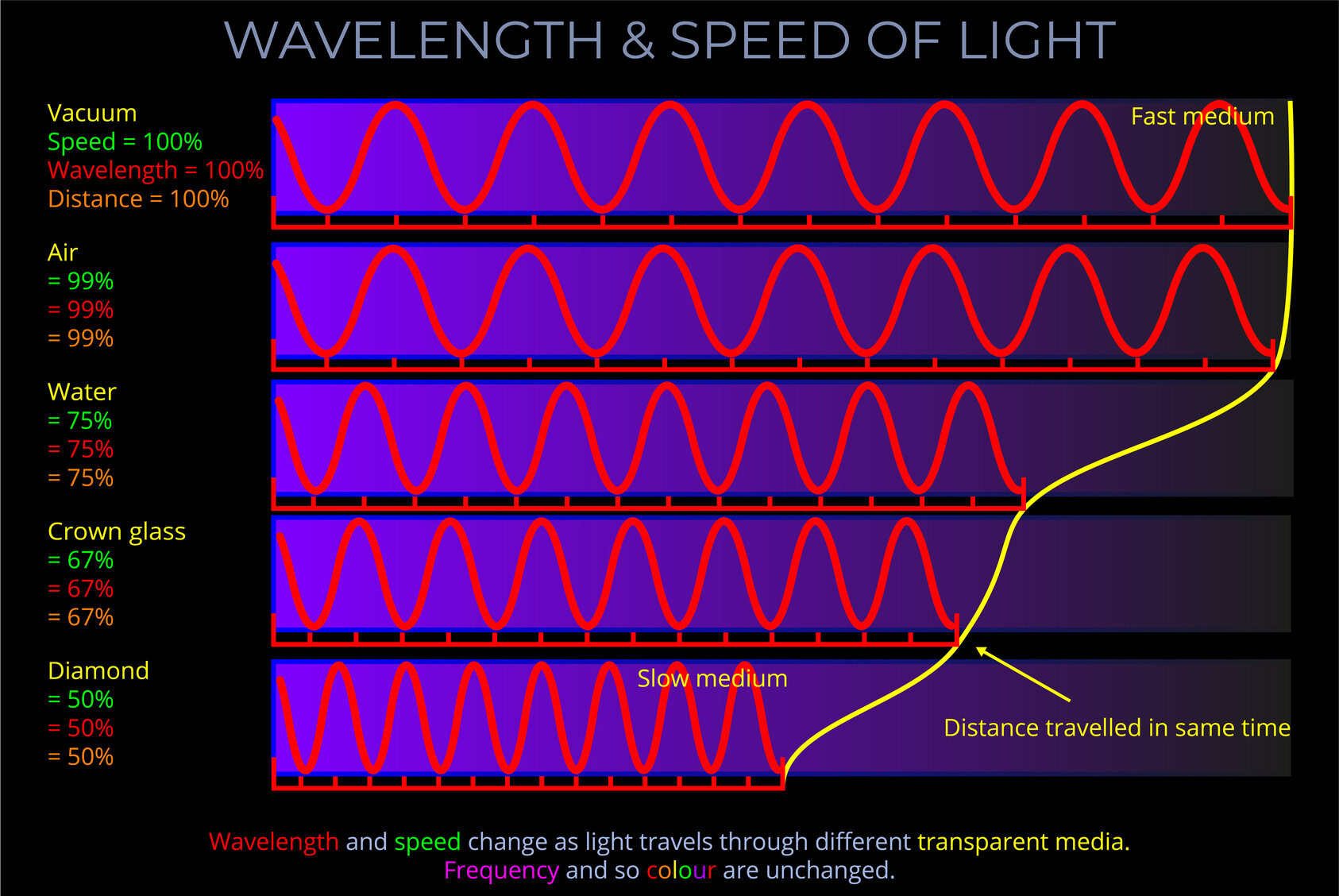
About speed & velocity
Speed and velocity are not the same. While they are related, there is a key difference between the two:
- Speed is a scalar quantity that refers to “how fast an object is moving.” It is the rate at which an object covers distance, without considering the direction of motion. It is expressed in units of distance per time, such as meters per second (m/s) or kilometres per hour (km/h).
- Velocity is a vector quantity that refers to “the rate at which an object changes its position.” It includes both the speed of the object and its direction of motion. It is also expressed in units of distance per time, such as meters per second (m/s) or kilometres per hour (km/h), but with a specified direction.
The Standard Model is a quantum field theory, which means it uses the principles of quantum mechanics to describe the behaviour of matter and energy at the atomic and subatomic levels.
- The Standard Model is based on two fundamental theories:
- Quantum mechanics describes the physical properties of nature as interactions between fields of energy at the scale of atoms and subatomic particles. It is the foundation of all quantum physics.
- Special relativity is a theory of space and time developed by Albert Einstein in 1905. It states that:
- The laws of physics are invariant (i.e., identical) in all inertial frames of reference.
- The speed of light in a vacuum is the same for all observers, regardless of the motion of the light source or observer.
Stellar light is the term used to describe the electromagnetic radiation emitted by stars, primarily due to the nuclear fusion of hydrogen atoms occurring within their cores.
- Unlike traditional sources of light on Earth, stars ignite with a far more powerful process – nuclear fusion.
- Deep within their incredibly dense and hot cores, immense pressure and temperatures fuel nuclear fusion.
- This process forces hydrogen atoms to merge into heavier elements, primarily helium, releasing tremendous energy.
- A fraction of this energy escapes the star as the radiant light we call sunlight and starlight.
The strong nuclear force is one of the four fundamental forces in nature. The other forces are the electromagnetic force, the weak nuclear force and gravity.
- The strong nuclear force is the strongest of the four fundamental forces of nature but only acts over very small distances, about the size of an atom’s nucleus. This short-range force is about 100 times stronger than the electromagnetic force, 106 times stronger than the weak nuclear force, and 1038 times stronger than gravity.
- The strong nuclear force is the fundamental force that binds matter together and is responsible for holding together protons and neutrons which are the subatomic particles within the atomic nucleus.
- The strong nuclear force counteracts the electrical repulsion between protons, which would otherwise push the positively charged protons apart.
- The strong nuclear force plays a crucial role in nuclear reactions, allowing the release of tremendous energy in processes like nuclear power generation and nuclear weapons.