- Here at lightcolourvision.org, we use the term ‘light’ as shorthand for ‘light of all wavelengths’ within the electromagnetic spectrum and so all forms of electromagnetic radiation. This includes:
- Radio waves (longest wavelength)
- Microwaves
- Infrared radiation
- Visible light
- Ultraviolet radiation
- X-rays
- Gamma rays (shortest wavelength)
light in classical physics
- Classical physics thinks of light as continuous waves. This means that a wave of light in the vacuum of space has a constant wavelength (so colour), frequency and brightness and that it propagates through space without any reduction in force or energy.
- This Classical view is applied in the sub-field of optics when dealing with things like the reflection, refraction and polarisation of light and when analysing the behaviour of lenses, mirrors, and lasers. Visible light can be described in terms of:
- Electromagnetic waves with wave-like properties including wavelength, frequency, amplitude, and phase.
- Particles called photons have both wave-like and particle-like properties.
- The electromagnetic wave theory of light is a key component of our understanding of visible light and its interactions with matter. It helps to explain phenomena such as reflection, refraction, and diffraction of light and plays a crucial role in technologies such as wireless communication, remote sensing and medical imaging.
light in quantum mechanics
- In the field of quantum mechanics, light is described as a stream of particles called photons, which are the quanta of the electromagnetic field. According to this theory, photons are massless particles of light that have no electric charge but have momentum and each photon constitutes a single packet of electromagnetic energy.
- One of the most famous experiments that demonstrate the particle-like nature of light is the photoelectric effect, in which electrons are emitted from a metal surface when exposed to certain wavelengths of light. The photoelectric effect can not be explained by the wave theory of light but is explained by Einstein’s theory of the photoelectric effect, which proposes that photons transfer their energy to electrons in the metal.
- The wave model and the quantum mechanical model of light are not mutually exclusive and can be used to develop different perspectives on the same phenomena.
- The wave model is useful for understanding light in situations where it behaves like a wave but largely ignores the way it interacts with matter at a sub-atomic scale.
- The quantum mechanical model of light is useful in understanding interactions between light and matter at a sub-atomic scale, particularly interactions involving single photons and other quantum particles such as electrons.
About light and colour
light
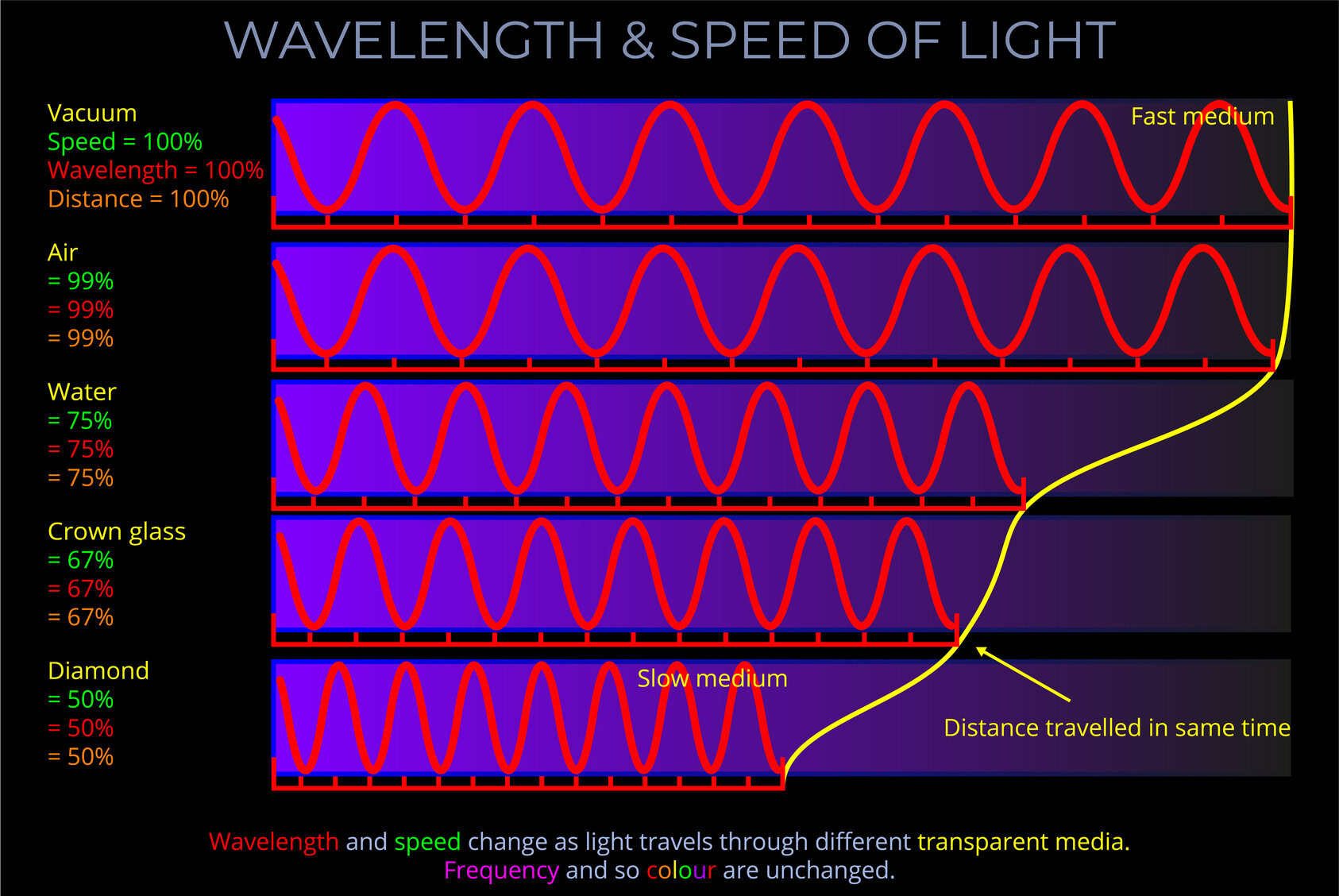
- Light travels at a speed of 299,792,458 meters per second in a vacuum, but its speed decreases when it passes through a medium rather than a vacuum.
- Light-matter interactions produce various optical phenomena such as absorption, dispersion, diffraction, polarization, reflection, refraction, scattering, and transmission.
- Light is electromagnetic radiation (radiant energy), which is transported by electromagnetic waves (or their quanta, photons) and travels through space.
light & colour
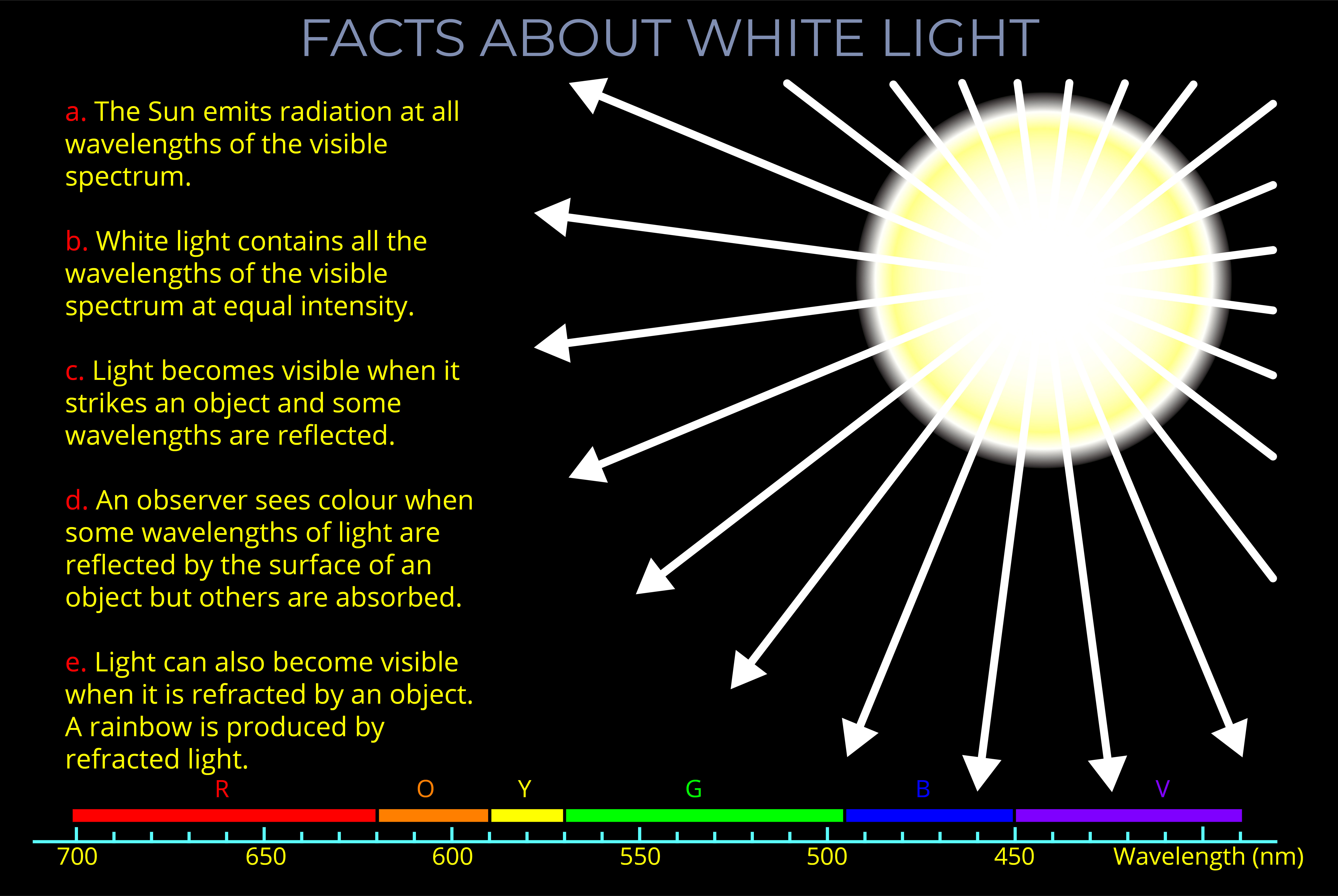
- Light and colour are related but distinct concepts. Light is a form of electromagnetic radiation, while colour is a perception that results from how the human eye and brain respond to different wavelengths of visible light.
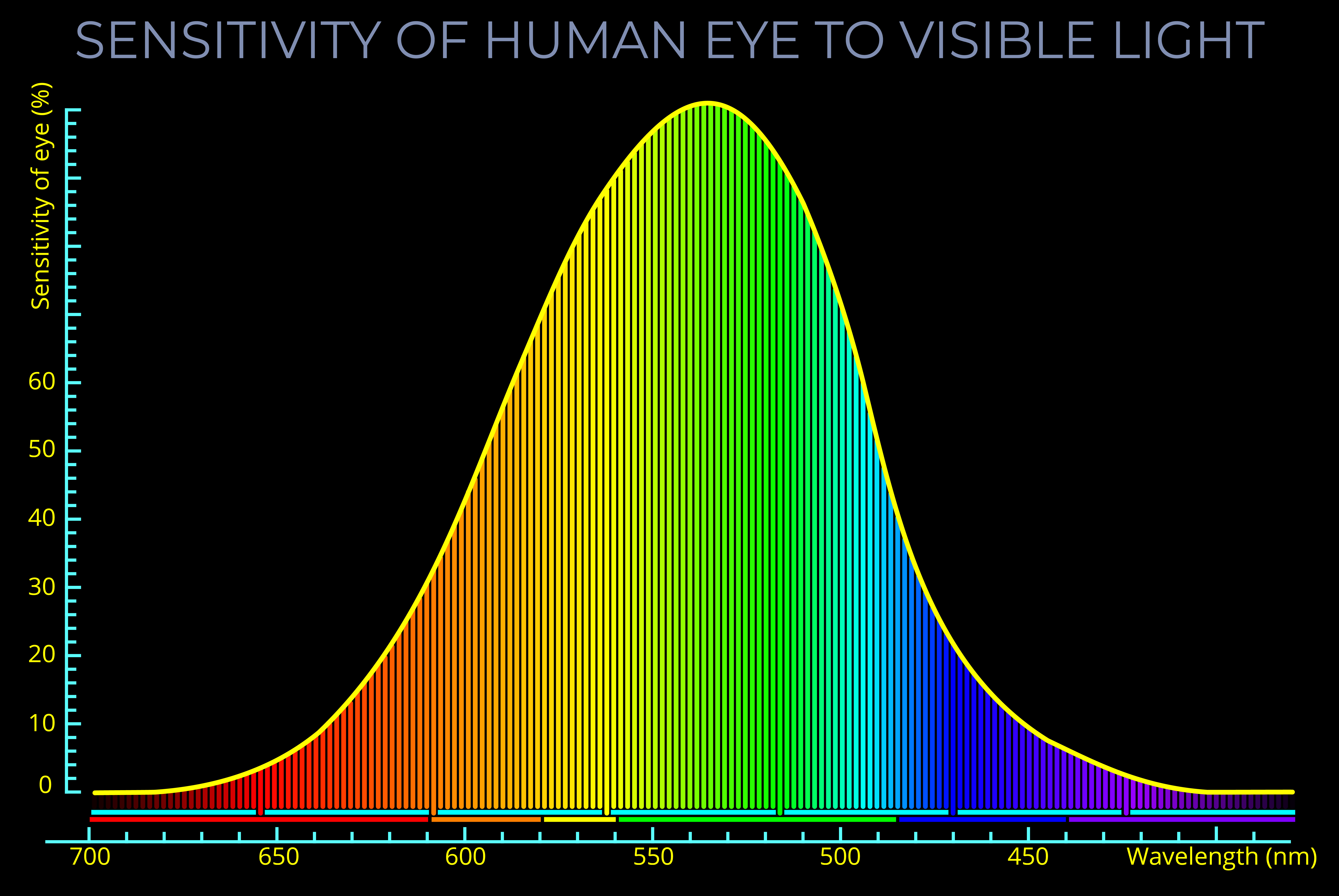
- The human eye can perceive only a small part of the electromagnetic spectrum, known as visible light, which includes wavelengths between about 400 and 700 nanometres.
- The perception of colour depends on the wavelengths of light that stimulate the cones in the retina.
- The perception of colour can vary among individuals and living organisms.
- Even if humans had never evolved, electromagnetic radiation would have been emitted by stars since the formation of the first galaxies over 13 billion years ago.
- Colour perception in humans primarily depends on the design of our eyes and the wavelength, frequency, and energy of the visible light that strikes the retina at the back of our eyes.
- Colour is a visual experience unique to each of us at any given moment because of our different points of view and perspectives on the world. So we share our experiences of colour using language to share our experiences of colour.
About light, radiation, radiant energy & electromagnetic energy
There is a difference in meaning between the terms light, electromagnetic radiation, radiant energy and electromagnetic energy in physics.
Light
-
- Light is best used to refer to the subset of electromagnetic radiation that is visible to the human eye, ranging from violet (shorter wavelengths) to red (longer wavelengths).
Electromagnetic radiation
-
- Electromagnetic radiation refers to the transfer of all forms of electromagnetic radiation through space by electromagnetic waves and includes gamma rays, ultraviolet (UV), infrared (IR), X-rays, and radio waves, as well as visible light.
- Electromagnetic radiation exhibits a wave-particle duality. This means it can behave like both a wave and a particle depending on the experiment or observation method.
Radiant energy
-
- Radiant energy is most commonly used to refer to electromagnetic radiation carried by electromagnetic waves and photons.
- Radiant energy can be measured using instruments such as photometers, which detect the intensity of light or other forms of electromagnetic radiation.
Electromagnetic energy
- Electromagnetic energy is a more general term that refers to any form of energy that is carried by electromagnetic waves, including both radiant energy and other types of energy that are not radiant (e.g., static electric fields).
- The type of energy associated with electromagnetic radiation is a measurable quantity in physics, and its measurement is essential for understanding and analyzing physical systems and processes.
- The unit of measurement for electromagnetic energy in the International System of Units (SI) is the joule (J), which is defined as the amount of energy required to perform one joule of work
- The electronvolt (eV) is another unit of energy commonly used in atomic and subatomic physics.
References
- https://en.wikipedia.org/wiki/Light