Wavelength Frequency & Energy Compared
£0.00
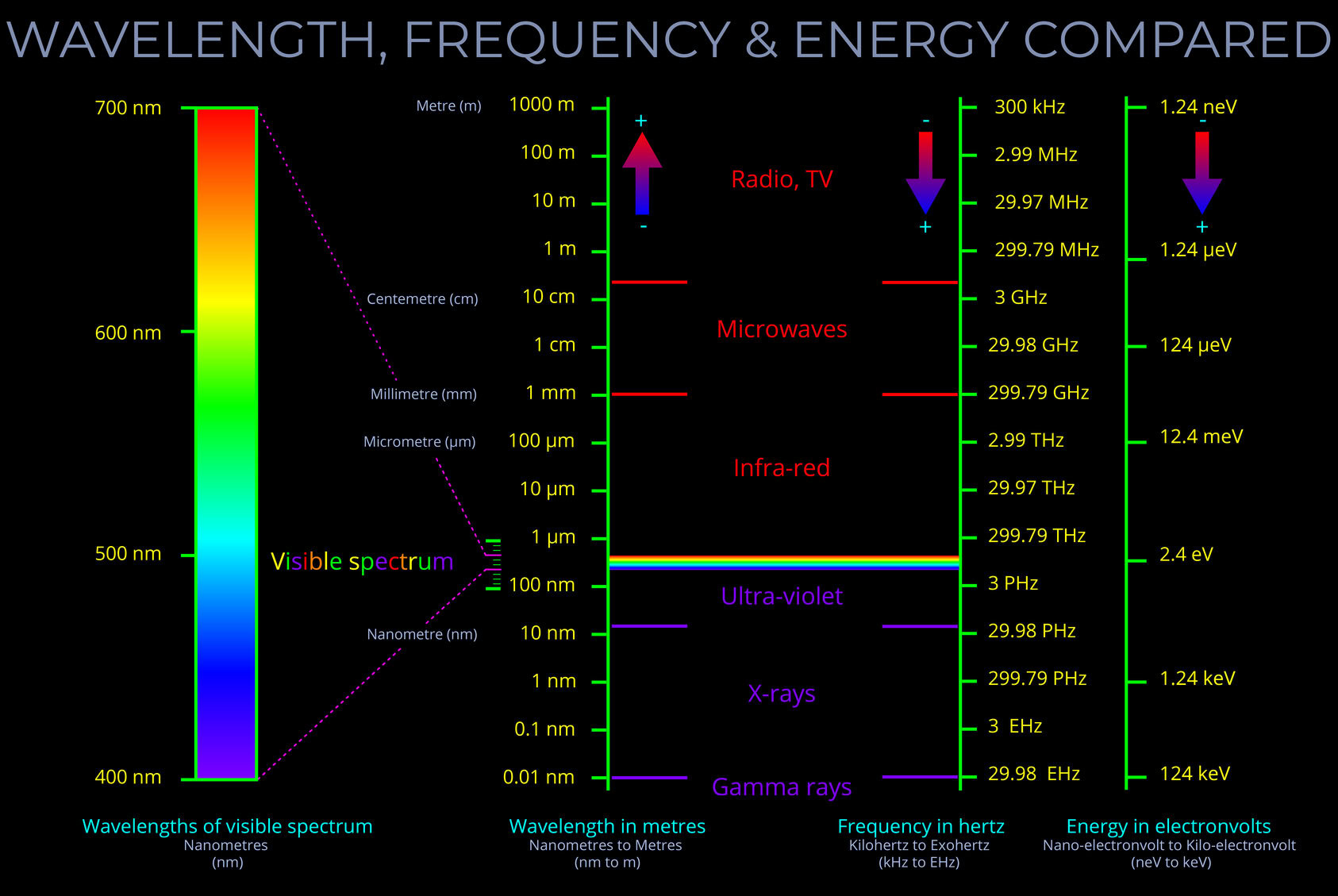
This diagram of the electromagnetic spectrum shows how wavelength, frequency and energy are related to one another.
- The diagram shows that the electromagnetic spectrum can be described as bands of electromagnetic radiation. Radio waves which have the longest wavelengths and the lowest frequency appear at the top of the diagram whilst gamma rays which have the shortest wavelengths but the highest frequencies appear at the bottom.
- A magnified view of the visible spectrum is shown on the left of the diagram. It forms a very small band of wavelengths, frequencies and energies within the electromagnetic spectrum as a whole.
- Compare wavelength, frequency and energy by reading across the three columns.
- For example, notice that in the wavelength column, the boundary between microwaves and radio waves is around 10 cm (centimetres). The corresponding value in the frequency column is 3GHz (gigahertz) and the energy column shows the energy carried by these waves as being between 1.24 µeV (micro electron volts) and 1.24 MeV (megaelectron volt).
Notice that:
- There are arrows in each column that show the longest wavelength in the wavelength column is at the top whilst the highest frequency and highest energies are at the bottom of their respective columns.
- The standard units in the three columns are metres, hertz and electronvolts, but metric prefixes are used to cope with the huge differences of scale from the top to the bottom of each column.
- Wavelength is inversely proportional to frequency and energy, so the arrow in the wavelength column faces in the opposite direction to the other two.
- Frequency and energy are directionally proportional so the arrows in those two columns face in the same direction.
Description
Wavelength, Frequency & Energy Compared
TRY SOME QUICK QUESTIONS AND ANSWERS TO GET STARTED
About the diagram
About the diagram
- This diagram of the electromagnetic spectrum shows how wavelength, frequency and energy are related to one another.
- The diagram shows that the electromagnetic spectrum can be described as bands of electromagnetic radiation. Radio waves which have the longest wavelengths and the lowest frequency appear at the top of the diagram whilst gamma rays which have the shortest wavelengths but the highest frequencies appear at the bottom.
- A magnified view of the visible spectrum is shown on the left of the diagram. It forms a very small band of wavelengths, frequencies and energies within the electromagnetic spectrum as a whole.
- Compare wavelength, frequency and energy by reading across the three columns.
- For example, notice that in the wavelength column, the boundary between microwaves and radio waves is around 10 cm (centimetres). The corresponding value in the frequency column is 3GHz (gigahertz) and the energy column shows the energy carried by these waves as being between 1.24 µeV (microelectron volts) and 1.24 MeV (megaelectron volt).
Notice that:
- There are arrows in each column that show the longest wavelength in the wavelength column is at the top whilst the highest frequency and highest energies are at the bottom of their respective columns.
- The standard units in the three columns are metres, hertz and electronvolts, but metric prefixes are used to cope with the huge differences of scale from the top to the bottom of each column.
- Wavelength is inversely proportional to frequency and energy, so the arrow in the wavelength column faces in the opposite direction to the other two.
- Frequency and energy are directionally proportional so the arrows in those two columns face in the same direction.
- The relationship between wavelength, frequency and energy means that:
- As the wavelength of an electromagnetic wave get shorter its frequency increases, and as the wavelength gets longer its frequency decreases.
- As the wavelength of an electromagnetic wave get shorter and its frequency increases and the amount of energy it transports becomes greater.
- As the energy transported by an electromagnetic wave increases so does its frequency whilst its wavelength gets shorter.
Some key terms
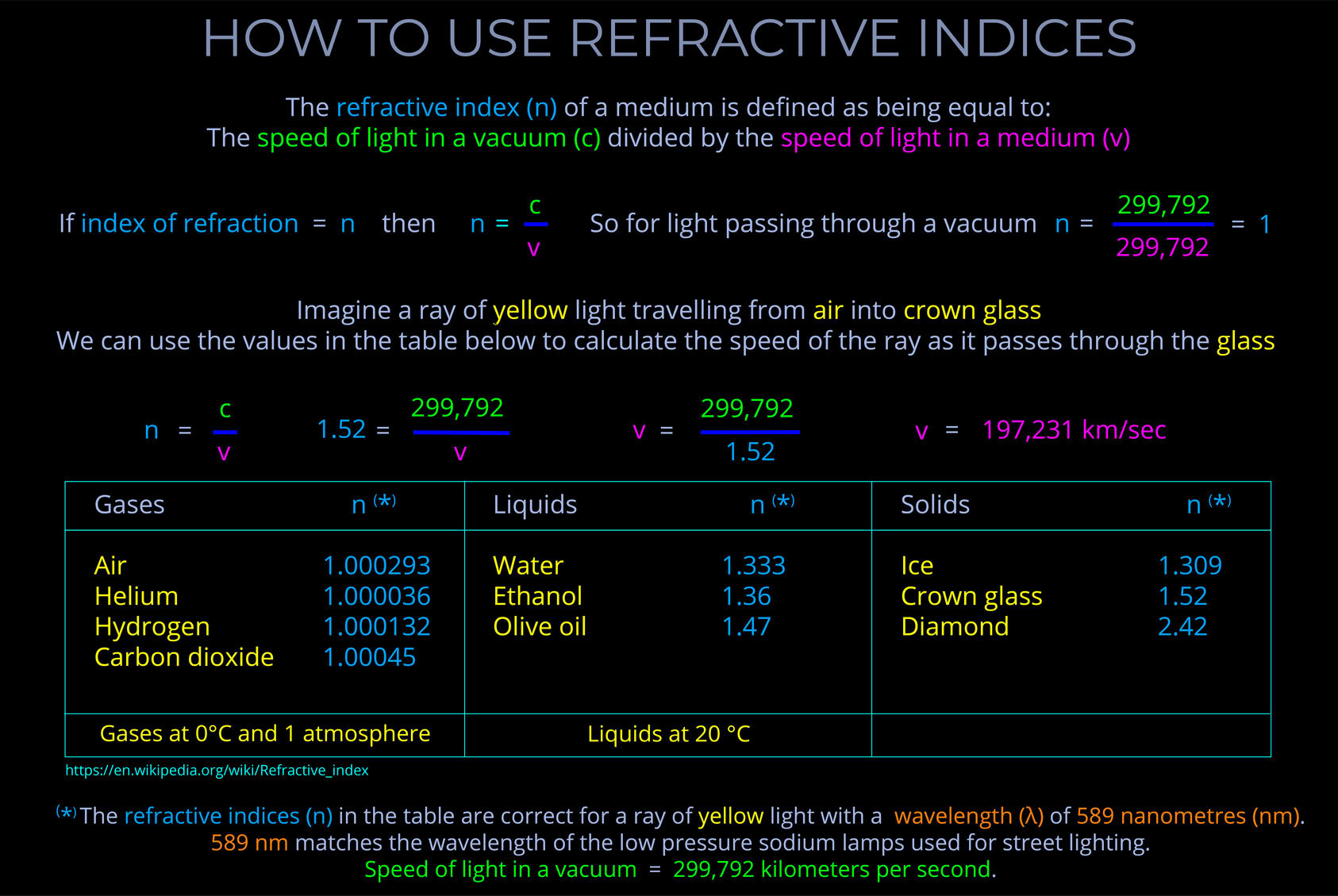
An electronvolt (eV) is a unit of energy commonly used in atomic, nuclear, and particle physics to measure the energy carried by individual particles and electromagnetic radiation. It’s a convenient unit because the energies involved in these fields are much smaller than those we encounter in everyday life.
- Electronvolts can be used to measure the energy of elementary particles, including photons, which are the smallest units of electromagnetic radiation (quanta of the electromagnetic field).
- The electronvolt is not part of the SI unit system. The SI unit for energy is the joule (J). However, joules are too large for many particle-level interactions.
- 1 electronvolt (eV) is equivalent to approximately 1.602 x 10^-19 joules (J).
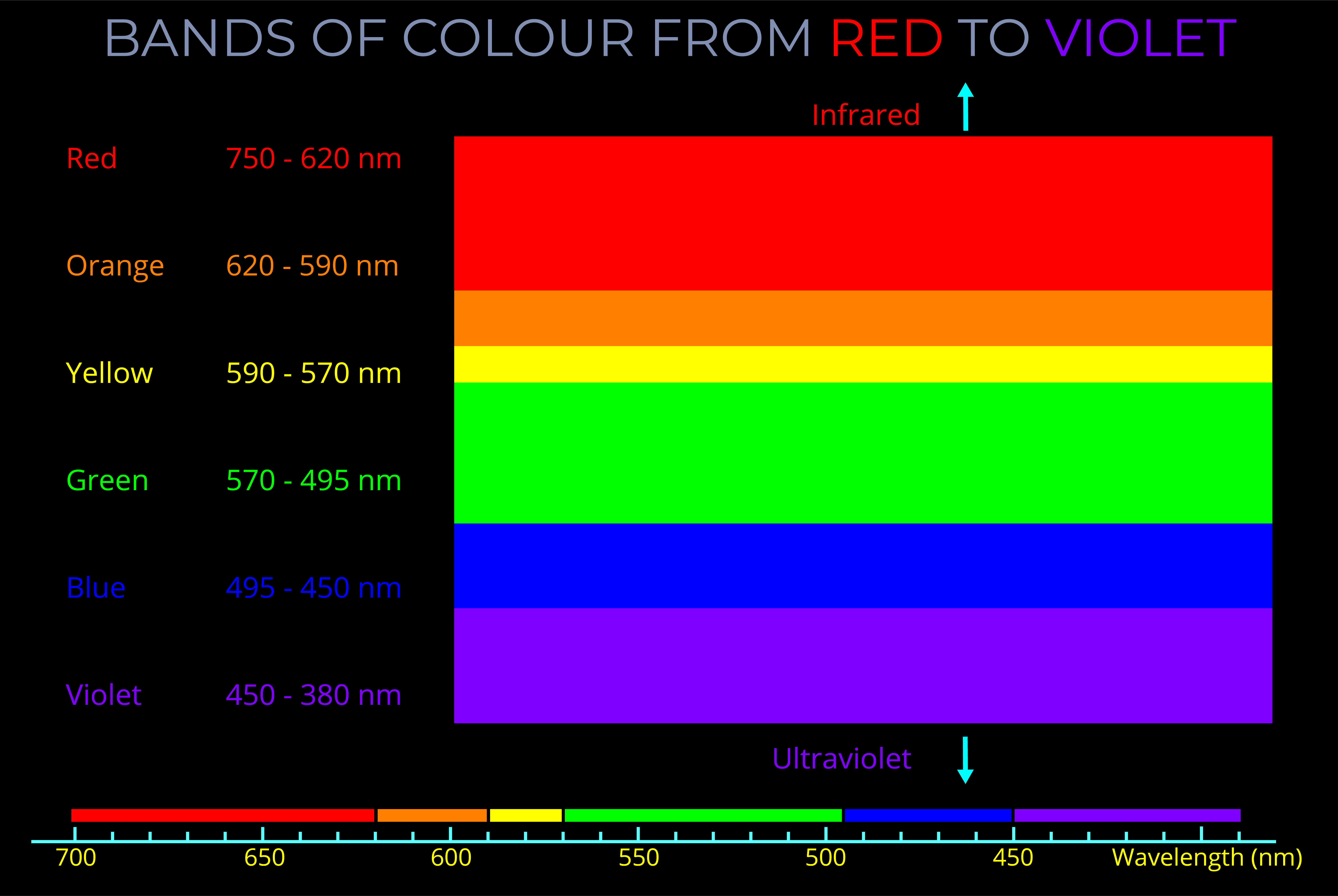
A nanometre (nm) is a unit of length in the metric system, equal to one billionth of a metre (1 nm = 1 × 10⁻⁹ metres). It is commonly used to measure extremely small distances, particularly at the atomic and molecular scale.
- In the context of light and electromagnetic radiation, a nanometre is often used to describe wavelengths of visible light.
The wavelength of visible light ranges from about 700 nm (red) to 400 nm (violet). - Nanometres are also used to measure components like the thickness of materials, the size of particles in nanotechnology, and the spacing between atoms in a crystal lattice.
Energy is a property of matter and fields, which can be transferred between systems or transformed into different forms but cannot be created or destroyed.
- Everything contains energy including all forms of matter and so all objects.
- Energy is evident in all forms of movement, interactions between, and changes to the forms and properties of matter.
- At an atomic level, energy is evident in the movement of electrons around the nucleus of an atom. Energy is stored in the nucleus of atoms as a result of the forces that bind protons and neutrons together.
- Energy can be transferred between objects, and converted from one form to another, but cannot be created or destroyed.
- Everything in the universe uses energy in one form or another.
- When it comes down to it, matter is energy.
- Light has energy but no mass so does not occupy space and has no volume.
- Energy is often described as either being potential energy or kinetic energy.
- Energy is measured in joules.
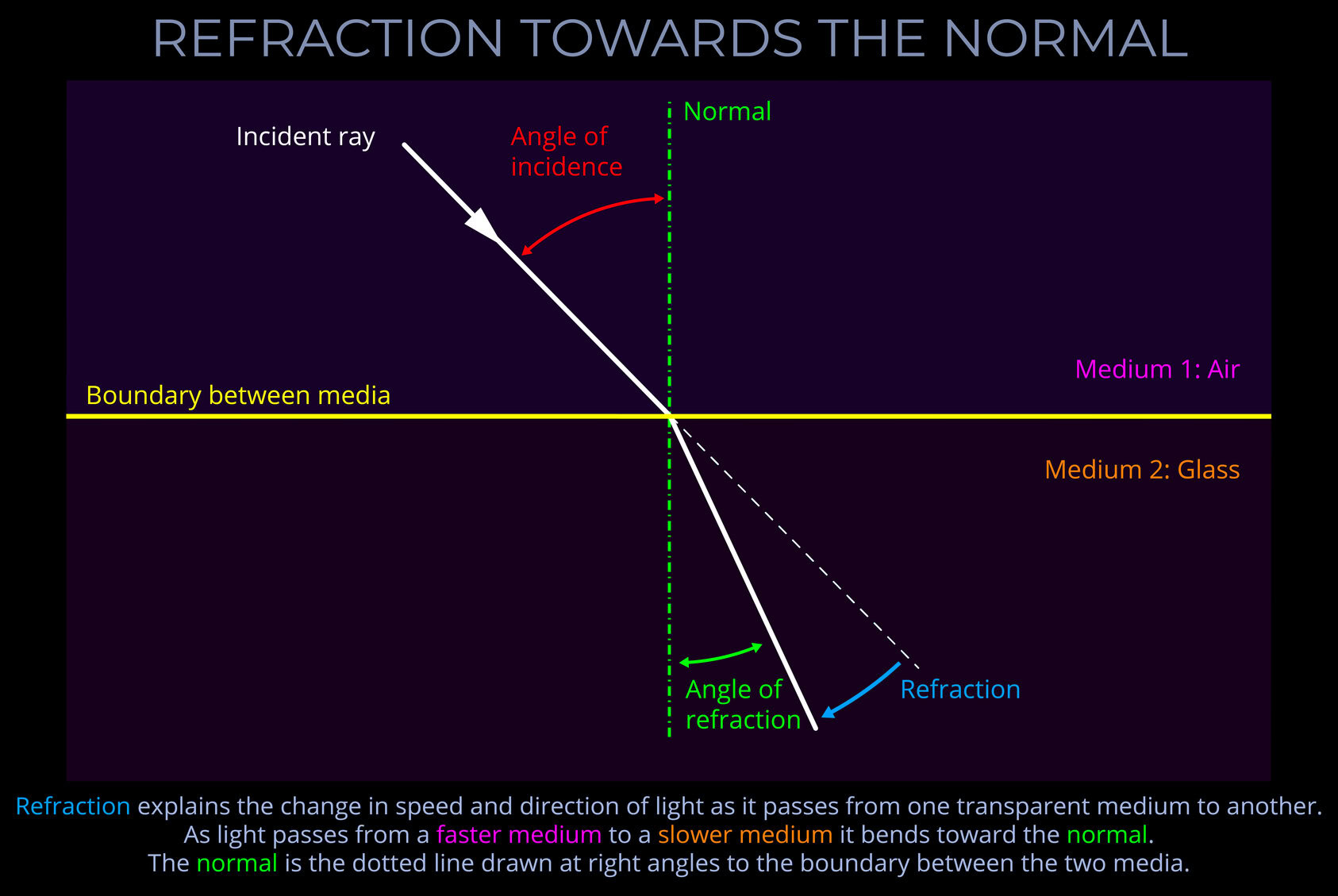
Wavelength measures a complete wave cycle, which is the distance from any point on a wave to the corresponding point on the next wave.
- While wavelength can be measured from any point on a wave, it is often simplest to measure from the peak of one wave to the peak of the next or from the bottom of one trough to the bottom of the next, ensuring the measurement covers the whole of the cycle.
- The wavelength of an electromagnetic wave is usually given in metres.
- The wavelength of visible light is typically measured in nanometres, with 1,000,000,000 nanometres making up a metre.
- Radio waves, visible light, and gamma waves for example, each have different ranges of wavelengths within the electromagnetic spectrum.
The hertz (symbol: Hz) is a unit used to measure the frequency of electromagnetic waves. It represents the number of wave-cycles per second.
- One hertz is defined as one cycle per second.
- Hertz measure the number of oscillations of the perpendicular electric and magnetic fields in electromagnetic radiation per second.
- Frequency conversions:
- 1 Hertz (Hz) = 1 cycle per second
- 1 Kilohertz (kHz) = 1,000 (thousand) cycles per second
- 1 Megahertz (MHz) = 1,000,000 (million) cycles per second
- 1 Gigahertz (GHz) = 1,000,000,000 (billion) cycles per second
- 1 Terahertz (THz) = 1,000,000,000,000 (trillion )cycles per second
The frequency of electromagnetic radiation (light) refers to the number of wave-cycles of an electromagnetic wave that pass a given point in a given amount of time.
- Frequency is measured in Hertz (Hz) and signifies the number of wave-cycles per second. Sub-units of Hertz enable measurements involving a higher count of wave-cycles within a single second.
- The frequency of electromagnetic radiation spans a broad range, from radio waves with low frequencies to gamma rays with high frequencies.
- The wavelength and frequency of light are closely linked. Specifically, as the wavelength becomes shorter, the frequency increases correspondingly.
- It is important not to confuse the frequency of a wave with the speed at which the wave travels or the distance it covers.
- The energy carried by a light wave intensifies as its oscillations increase in number and its wavelength shortens.