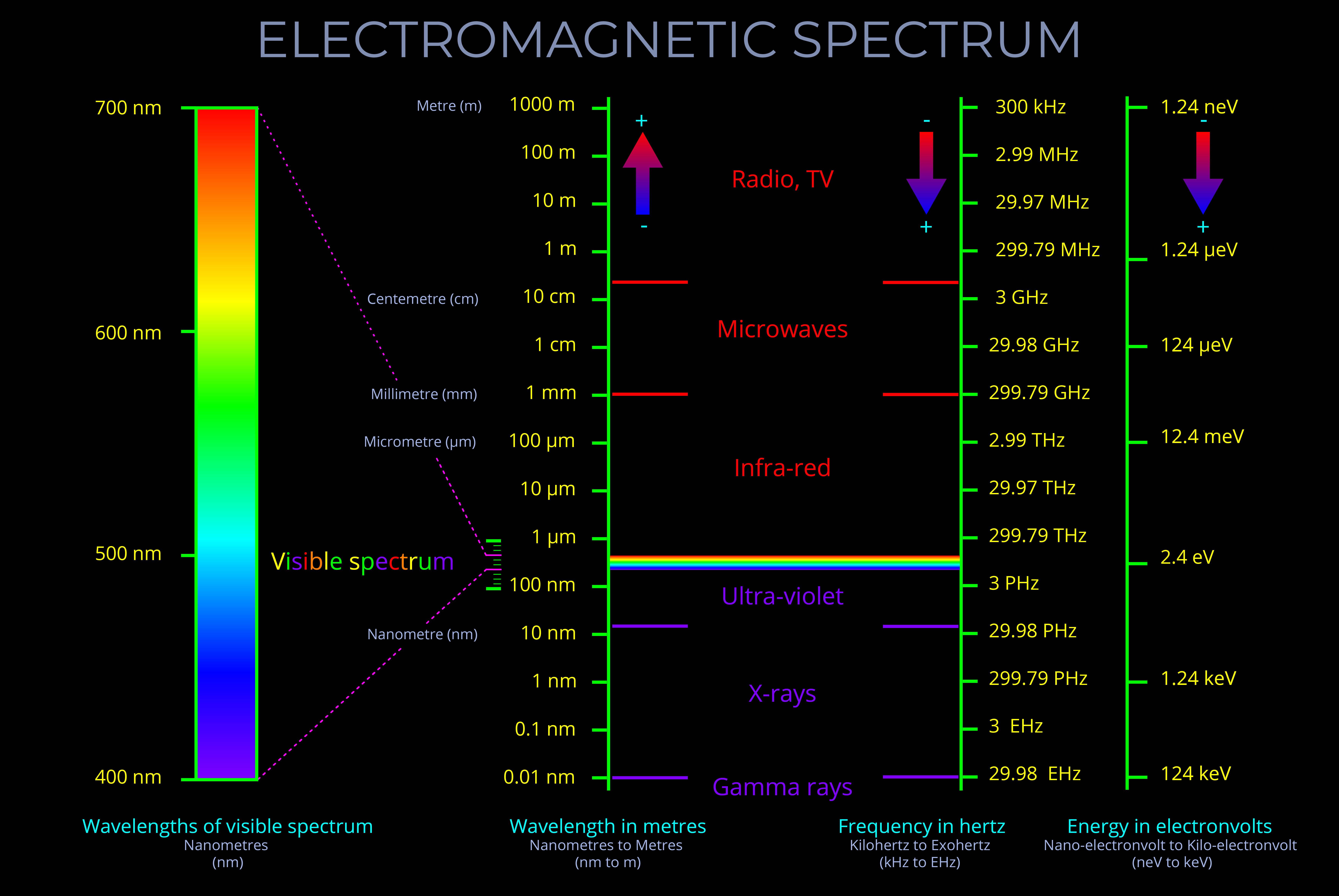
The electromagnetic spectrum encompasses electromagnetic waves with every possible wavelength (and corresponding frequencies) of electromagnetic radiation, spanning low-energy radio waves through visible light to high-energy gamma rays.
- The electromagnetic spectrum is continuous, with no gaps between the different types of waves.
- There are no precisely defined boundaries between the bands of electromagnetic radiation in the electromagnetic spectrum.
- The electromagnetic spectrum includes, in order of increasing frequency: radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays and gamma rays.
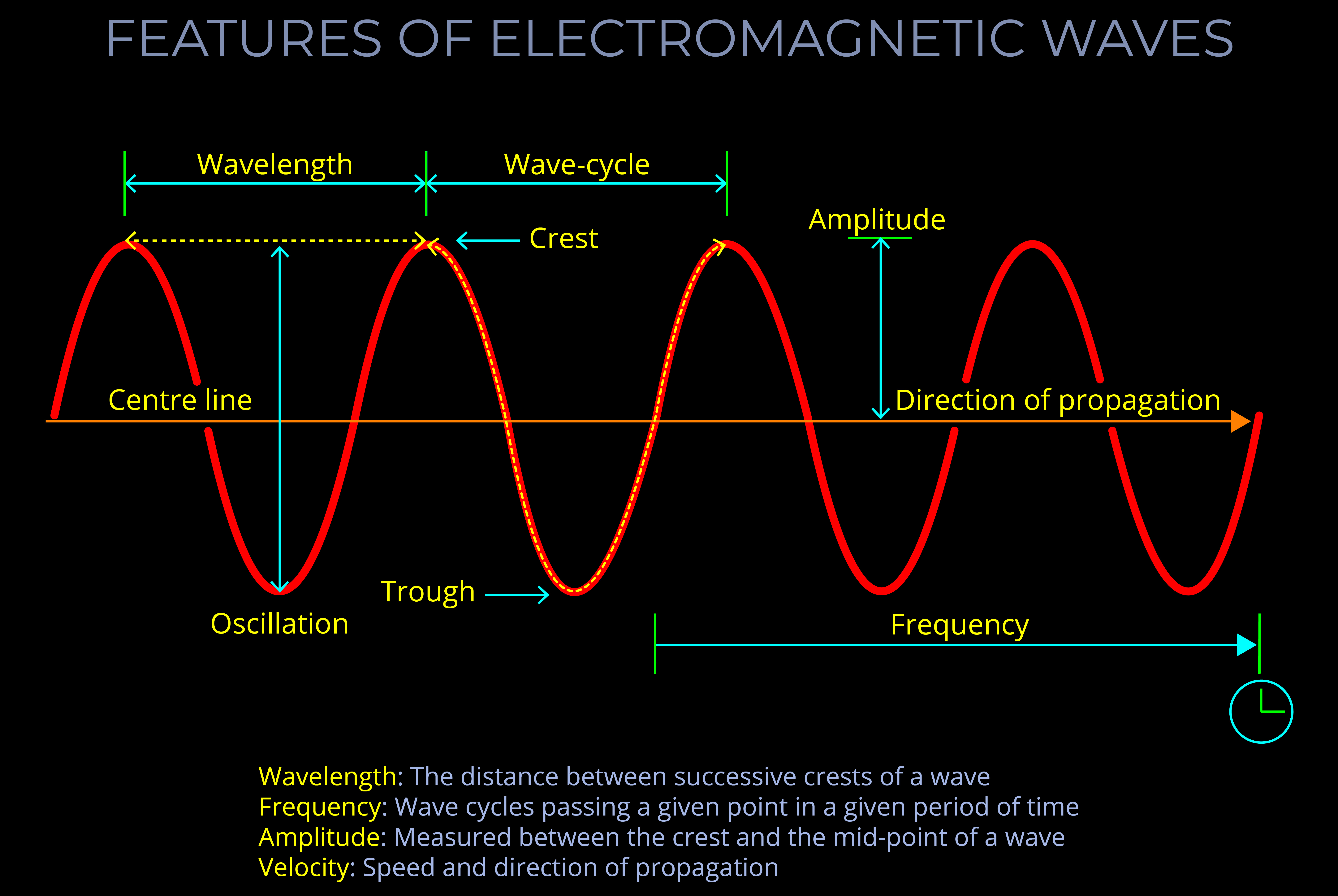
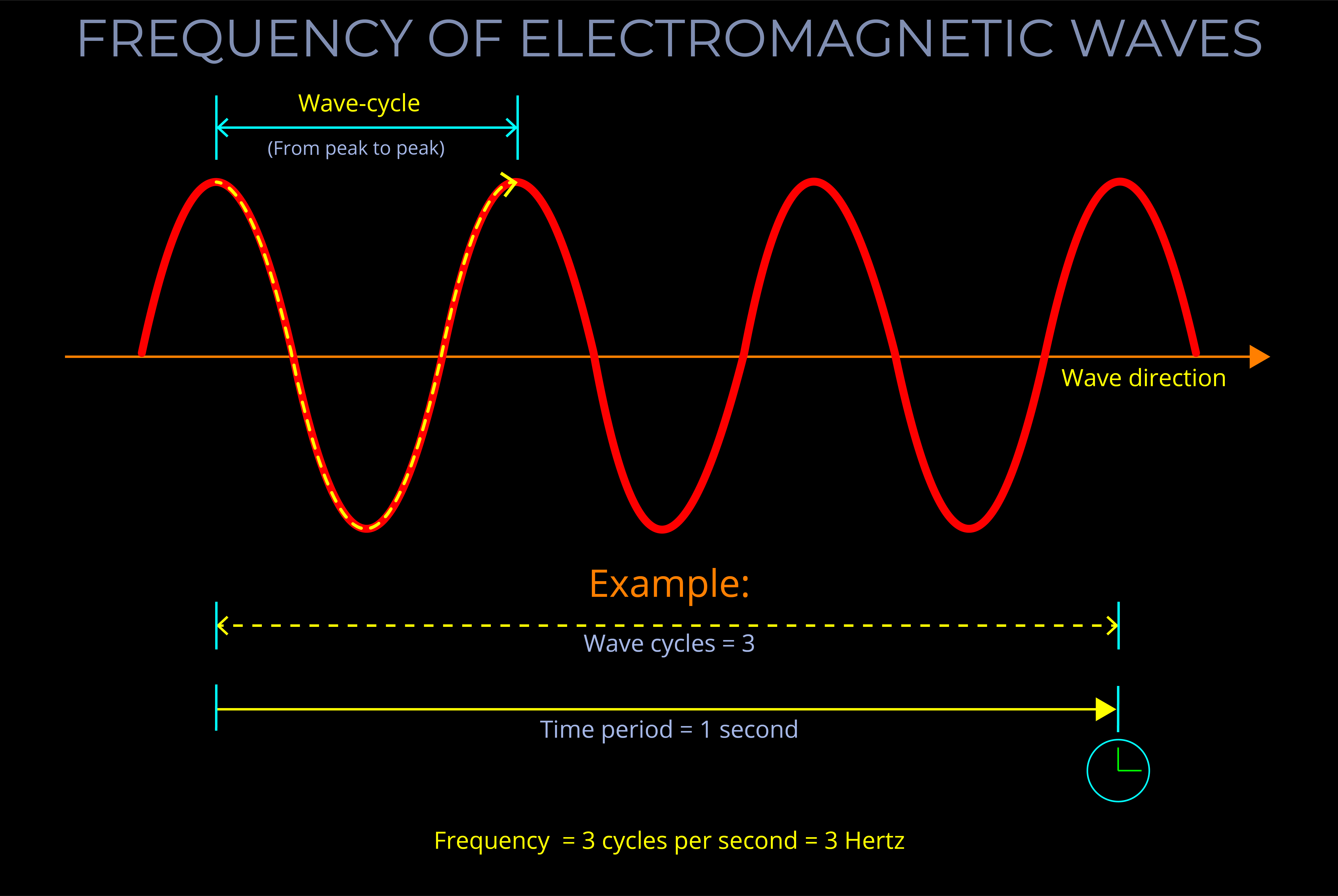
- The frequency of an electromagnetic wave is inversely proportional to its wavelength. This means that as the frequency increases, the wavelength decreases, and vice versa.
- Visible light constitutes only a small fraction of the entire electromagnetic spectrum.
- Each type of electromagnetic wave has distinct properties and interacts differently with matter.
- Electromagnetic waves can carry energy, momentum, and angular momentum away from their source and can impart those quantities to matter they interact with.
About the generation of electromagnetic waves
Oscillating Charges
- When electric charges oscillate, they produce varying electric fields. This change in the electric field also produces a changing magnetic field. These varying electric and magnetic fields generate an electromagnetic wave that propagates away from its source.
Electromagnetic Induction
- Electromagnetic waves can be generated by changing magnetic fields. When a magnetic field alters, it induces an electric field. This fluctuating electric field in turn produces a varying magnetic field, resulting in an electromagnetic wave.
Acceleration of Charged Particles
- When a charged particle is accelerated, it emits electromagnetic radiation. This principle is used in devices such as radio antennas and television broadcasting systems, where electrons are rapidly accelerated in metallic structures, producing radio and television waves.
Thermal Emission
- All objects emit electromagnetic radiation due to the thermal motion of charged particles within them. This thermal radiation increases in intensity and shifts to shorter wavelengths as the temperature of the object increases. An everyday example of this is the glow of red-hot or white-hot objects.
Electronic Transitions in Atoms and Molecules
- When electrons in atoms or molecules transition between energy levels (either absorbing energy to move to a higher energy level or emitting energy to fall to a lower energy level), they emit or absorb electromagnetic waves.
Nuclear Transitions
- Similar to electronic transitions, nuclear transitions (changes in energy levels within atomic nuclei) also produce electromagnetic radiation, typically in the gamma-ray region of the spectrum.
Annihilation Processes
- When a particle and its corresponding antiparticle collide, they annihilate each other, and their rest mass is converted into electromagnetic radiation, typically in the form of gamma rays.